Modulation of ISWI function by site-specific histone acetylation
- PMID: 11882543
- PMCID: PMC1084020
- DOI: 10.1093/embo-reports/kvf056
Modulation of ISWI function by site-specific histone acetylation
Abstract
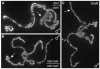
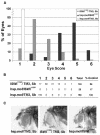
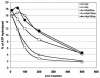
Mutations in Drosophila ISWI, a member of the SWI2/SNF2 family of chromatin remodeling ATPases, alter the global architecture of the male X chromosome. The transcription of genes on this chromosome is increased 2-fold relative to females due to dosage compensation, a process involving the acetylation of histone H4 at lysine 16 (H4K16). Here we show that blocking H4K16 acetylation suppresses the X chromosome defects resulting from loss of ISWI function in males. In contrast, the forced acetylation of H4K16 in ISWI mutant females causes X chromosome defects indistinguishable from those seen in ISWI mutant males. Increased expression of MOF, the histone acetyltransferase that acetylates H4K16, strongly enhances phenotypes resulting from the partial loss of ISWI function. Peptide competition assays revealed that H4K16 acetylation reduces the ability of ISWI to interact productively with its substrate. These findings suggest that H4K16 acetylation directly counteracts chromatin compaction mediated by the ISWI ATPase.
Figures

References
-
- Akhtar A. and Becker, P.B. (2000) Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol. Cell, 5, 367–375. - PubMed
-
- Bashaw G.J. and Baker, B.S. (1995) The msl-2 dosage compensation gene of Drosophila encodes a putative DNA-binding protein whose expression is sex specifically regulated by Sex-lethal. Development, 121, 3245–3258. - PubMed
-
- Blank T.A. and Becker, P.B. (1995) Electrostatic mechanism of nucleosome spacing. J. Mol. Biol., 252, 305–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

