Mutation of Walker-A lysine 464 in cystic fibrosis transmembrane conductance regulator reveals functional interaction between its nucleotide-binding domains
- PMID: 11882668
- PMCID: PMC2290141
- DOI: 10.1113/jphysiol.2001.013162
Mutation of Walker-A lysine 464 in cystic fibrosis transmembrane conductance regulator reveals functional interaction between its nucleotide-binding domains
Abstract
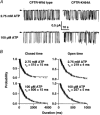
The cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel bears two nucleotide-binding domains (NBD1 and NBD2) that control its ATP-dependent gating. Exactly how these NBDs control gating is controversial. To address this issue, we examined channels with a Walker-A lysine mutation in NBD1 (K464A) using the patch clamp technique. K464A mutants have an ATP dependence (EC(50) approximate 60 microM) and opening rate at 2.75 mM ATP (approximately 2.1 s(-1)) similar to wild type (EC(50) approximate 97 microM; approximately 2.0 s(-1)). However, K464A's closing rate at 2.75 mM ATP (approximately 3.6 s(-1)) is faster than that of wild type (approximately 2.1 s(-1)), suggesting involvement of NBD1 in nucleotide-dependent closing. Delay of closing in wild type by adenylyl imidodiphosphate (AMP-PNP), a non-hydrolysable ATP analogue, is markedly diminished in K464A mutants due to reduction in AMP-PNP's apparent on-rate and acceleration of its apparent off-rate (approximately 2- and approximately 10-fold, respectively). Since the delay of closing by AMP-PNP is thought to occur via NBD2, K464A's effect on the NBD2 mutant K1250A was examined. In sharp contrast to K464A, K1250A single mutants exhibit reduced opening (approximately 0.055 s(-1)) and closing (approximately 0.006 s(-1)) rates at millimolar [ATP], suggesting a role for K1250 in both opening and closing. At millimolar [ATP], K464A-K1250A double mutants close approximately 5-fold faster (approximately 0.029 s(-1)) than K1250A but open with a similar rate (approximately 0.059 s(-1)), indicating an effect of K464A on NBD2 function. In summary, our results reveal that both of CFTR's functionally asymmetric NBDs participate in nucleotide-dependent closing, which provides important constraints for NBD-mediated gating models.
Figures



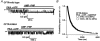




Comment in
-
The perplexing challenges of a pump turned channel.J Physiol. 2002 Mar 1;539(Pt 2):331. doi: 10.1113/jphysiol.2002.017210. J Physiol. 2002. PMID: 11882666 Free PMC article. No abstract available.
Similar articles
-
On the mechanism of MgATP-dependent gating of CFTR Cl- channels.J Gen Physiol. 2003 Jan;121(1):17-36. doi: 10.1085/jgp.20028673. J Gen Physiol. 2003. PMID: 12508051 Free PMC article.
-
Gating of cystic fibrosis transmembrane conductance regulator chloride channels by adenosine triphosphate hydrolysis. Quantitative analysis of a cyclic gating scheme.J Gen Physiol. 1999 Apr;113(4):541-54. doi: 10.1085/jgp.113.4.541. J Gen Physiol. 1999. PMID: 10102935 Free PMC article.
-
The two ATP binding sites of cystic fibrosis transmembrane conductance regulator (CFTR) play distinct roles in gating kinetics and energetics.J Gen Physiol. 2006 Oct;128(4):413-22. doi: 10.1085/jgp.200609622. Epub 2006 Sep 11. J Gen Physiol. 2006. PMID: 16966475 Free PMC article.
-
ATP hydrolysis cycles and the gating of CFTR Cl- channels.Acta Physiol Scand Suppl. 1998 Aug;643:247-56. Acta Physiol Scand Suppl. 1998. PMID: 9789567 Review.
-
STRUCTURE, GATING, AND REGULATION OF THE CFTR ANION CHANNEL.Physiol Rev. 2019 Jan 1;99(1):707-738. doi: 10.1152/physrev.00007.2018. Physiol Rev. 2019. PMID: 30516439 Review.
Cited by
-
Thermodynamics of CFTR channel gating: a spreading conformational change initiates an irreversible gating cycle.J Gen Physiol. 2006 Nov;128(5):523-33. doi: 10.1085/jgp.200609558. Epub 2006 Oct 16. J Gen Physiol. 2006. PMID: 17043148 Free PMC article.
-
The perplexing challenges of a pump turned channel.J Physiol. 2002 Mar 1;539(Pt 2):331. doi: 10.1113/jphysiol.2002.017210. J Physiol. 2002. PMID: 11882666 Free PMC article. No abstract available.
-
On the mechanism of MgATP-dependent gating of CFTR Cl- channels.J Gen Physiol. 2003 Jan;121(1):17-36. doi: 10.1085/jgp.20028673. J Gen Physiol. 2003. PMID: 12508051 Free PMC article.
-
The ABC protein turned chloride channel whose failure causes cystic fibrosis.Nature. 2006 Mar 23;440(7083):477-83. doi: 10.1038/nature04712. Nature. 2006. PMID: 16554808 Free PMC article. Review.
-
Vx-770 potentiates CFTR function by promoting decoupling between the gating cycle and ATP hydrolysis cycle.Proc Natl Acad Sci U S A. 2013 Mar 12;110(11):4404-9. doi: 10.1073/pnas.1215982110. Epub 2013 Feb 25. Proc Natl Acad Sci U S A. 2013. PMID: 23440202 Free PMC article.
References
-
- Alberts P, Daumke O, Deverson EV, Howard JC, Knittler MR. Distinct functional properties of the TAP subunits coordinate the nucleotide-dependent transport cycle. Current Biology. 2001;11:242–251. - PubMed
-
- Aleksandrov L, Mengos A, Chang X, Aleksandrov A, Riordan JR. Differential interactions of nucleotides at the two nucleotide binding domains of the cystic fibrosis transmembrane conductance regulator. Journal of Biological Chemistry. 2001;276:12918–12923. - PubMed
-
- Anderson MP, Berger HA, Rich DP, Gregory RJ, Smith AE, Welsh MJ. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 1991;67:775–784. - PubMed
-
- Anderson MP, Welsh MJ. Regulation by ATP and ADP of CFTR chloride channels that contain mutant nucleotide-binding domains. Science. 1992;257:1701–1704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

