Prostaglandin E(2) inhibits calcium current in two sub-populations of acutely isolated mouse trigeminal sensory neurons
- PMID: 11882676
- PMCID: PMC2290145
- DOI: 10.1113/jphysiol.2001.013322
Prostaglandin E(2) inhibits calcium current in two sub-populations of acutely isolated mouse trigeminal sensory neurons
Abstract
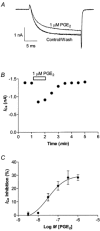
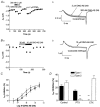
Prostaglandins are important mediators of pain and inflammation. We have examined the effects of prostanoids on voltage-activated calcium currents (I(Ca)) in acutely isolated mouse trigeminal sensory neurons, using standard whole cell voltage clamp techniques. Trigeminal neurons were divided into two populations based on the presence (Type 2) or absence (Type 1) of low voltage-activated T-type I(Ca). The absence of T-type I(Ca) is highly correlated with sensitivity to mu-opioid agonists and the VR1 agonist capsaicin. In both populations of cells, high voltage-activated I(Ca) was inhibited by PGE(2) with an EC(50) of about 35 nM, to a maximum of 30 %. T-type I(Ca) was not inhibited by PGE(2). Pertussis toxin pre-treatment abolished the effects of PGE(2) in Type 2 cells, but not in Type 1 cells, whereas treatment with cholera toxin prevented the effects of PGE(2) in Type 1 cells, but not in Type 2 cells. Inhibition of I(Ca) by PGE(2) was associated with slowing of current activation and could be relieved with a large positive pre-pulse, consistent with inhibition of I(Ca) by G protein betagamma subunits. Reverse transcription-polymerase chain reaction of mRNA from trigeminal ganglia indicated that all four EP prostanoid receptors were present. However, in both Type 1 and Type 2 cells the effects of PGE(2) were only mimicked by the selective EP(3) receptor agonist ONO-AE-248, and not by selective agonists for EP(1) (ONO-DI-004), EP(2) (ONO-AE1-259) and EP(4) (ONO-AE1-329) receptors. These data indicate that two populations of neurons in trigeminal ganglia differing in their calcium channel expression, sensitivity to mu-opioids and capsaicin also have divergent mechanisms of PGE(2)-mediated inhibition of calcium channels, with Gi/Go type G proteins involved in one population, and Gs type G proteins in the other.
Figures





References
-
- Bourinet E, Soong TW, Sutton K, Slaymaker S, Mathews E, Monteil A, Zamponi GW, Nargeot J, Snutch TP. Splicing of a1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nature Neuroscience. 1999;2:407–421. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

