Dissociation of the store-operated calcium current I(CRAC) and the Mg-nucleotide-regulated metal ion current MagNuM
- PMID: 11882677
- PMCID: PMC2290162
- DOI: 10.1113/jphysiol.2001.013361
Dissociation of the store-operated calcium current I(CRAC) and the Mg-nucleotide-regulated metal ion current MagNuM
Abstract
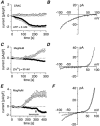
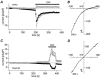
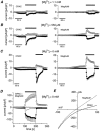
Rat basophilic leukaemia cells (RBL-2H3-M1) were used to study the characteristics of the store-operated Ca(2+) release-activated Ca(2+) current (I(CRAC)) and the magnesium-nucleotide-regulated metal cation current (MagNuM) (which is conducted by the LTRPC7 channel). Pipette solutions containing 10 mM BAPTA and no added ATP induced both currents in the same cell, but the time to half-maximal activation for MagNuM was about two to three times slower than that of I(CRAC). Differential suppression of I(CRAC) was achieved by buffering free [Ca(2+)](i) to 90 nM and selective inhibition of MagNuM was accomplished by intracellular solutions containing 6 mM Mg.ATP, 1.2 mM free [Mg(2+)](i) or 100 microM GTP-gamma-S, allowing investigations on these currents in relative isolation. Removal of extracellular Ca(2+) and Mg(2+) caused both currents to be carried significantly by monovalent ions. In the absence or presence of free [Mg(2+)](i), I(CRAC) carried by monovalent ions inactivated more rapidly and more completely than MagNuM carried by monovalent ions. Since several studies have used divalent-free solutions on either side of the membrane to study selectivity and single-channel behaviour of I(CRAC), these experimental conditions would have favoured the contribution of MagNuM to monovalent conductance and call for caution in interpreting results where both I(CRAC) and MagNuM are activated.
Figures

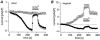

References
-
- Braun FJ, Broad LM, Armstrong DL, Putney JW., Jr Stable activation of single CRAC-channels in divalent cation-free solutions. Journal of Biological Chemistry. 2000;276:1063–1070. - PubMed
-
- Broad LM, Armstrong DL, Putney JW. Role of the inositol 1,4,5-trisphosphate receptor in Ca2+ feedback Inhibition of calcium release-activated calcium current ICRAC. Journal of Biological Chemistry. 1999;274:32881–32888. - PubMed
-
- Fasolato C, Hoth M, Penner R. A GTP-dependent step in the activation mechanism of capacitative calcium influx. Journal of Biological Chemistry. 1993;268:20737–20740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

