CD59 blocks not only the insertion of C9 into MAC but inhibits ion channel formation by homologous C5b-8 as well as C5b-9
- PMID: 11882685
- PMCID: PMC2290142
- DOI: 10.1113/jphysiol.2001.013381
CD59 blocks not only the insertion of C9 into MAC but inhibits ion channel formation by homologous C5b-8 as well as C5b-9
Abstract
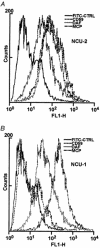
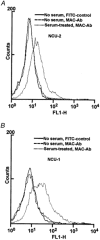
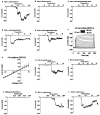
Activation of the complement system on the cell surface results in the insertion of pore forming membrane attack complexes (MAC, C5b-9). In order to protect themselves from the complement attack, the cells express several regulatory molecules, including the terminal complex regulator CD59 that inhibits assembly of the large MACs by inhibiting the insertion of additional C9 molecules into the C5b-9 complex. Using the whole cell patch clamp method, we were able to measure accumulation of homologous MACs in the membrane of CD59(-) human B-cells, which formed non-selective ion channels with a total conductance of 360 +/- 24 pS as measured at the beginning of the steady-state phase of the inward currents. C5b-8 and small-size MAC (MAC containing only a single C9) can also form ion channels. Nevertheless, in CD59(+) human B-cells in spite of small-size MAC formation, an ion current could not be detected. In addition, restoring CD59 to the membrane of the CD59(-) cells inhibited the serum-evoked inward current. The ion channels formed by the small-size MAC were therefore sealed, indicating that CD59 directly interfered with the pore formation of C5b-8 as well as that of small-size C5b-9. These results offer an explanation as to why CD59-expressing cells are not leaky in spite of a buildup of homologous C5b-8 and small-size MAC. Our experiments also confirmed that ion channel inhibition by CD59 is subject to homologous restriction and that CD59 cannot block the conductivity of MAC when generated by xenogenic (rabbit) serum.
Figures
Similar articles
-
Inhibition of homologous complement by CD59 is mediated by a species-selective recognition conferred through binding to C8 within C5b-8 or C9 within C5b-9.J Immunol. 1991 Apr 1;146(7):2345-51. J Immunol. 1991. PMID: 1706395
-
Interactions of soluble CD59 with the terminal complement complexes. CD59 and C9 compete for a nascent epitope on C8.J Immunol. 1993 Nov 1;151(9):4941-9. J Immunol. 1993. PMID: 7691959
-
The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9.J Immunol. 1990 May 1;144(9):3478-83. J Immunol. 1990. PMID: 1691760
-
Transmembrane channel-formation by five complement proteins.Biochem Soc Symp. 1985;50:235-46. Biochem Soc Symp. 1985. PMID: 2428370 Review.
-
The killer molecule of complement.J Invest Dermatol. 1985 Jul;85(1 Suppl):47s-52s. doi: 10.1111/1523-1747.ep12275445. J Invest Dermatol. 1985. PMID: 3891882 Review.
Cited by
-
Complement Membrane Attack Complex: New Roles, Mechanisms of Action, and Therapeutic Targets.Am J Pathol. 2020 Jun;190(6):1138-1150. doi: 10.1016/j.ajpath.2020.02.006. Epub 2020 Mar 16. Am J Pathol. 2020. PMID: 32194049 Free PMC article. Review.
-
New insights into the reaction of Schistosoma mansoni cercaria to the human complement system.Parasitol Res. 2014 Oct;113(10):3685-96. doi: 10.1007/s00436-014-4033-3. Epub 2014 Jul 17. Parasitol Res. 2014. PMID: 25030119 Free PMC article.
-
Immune Protection of Retroviral Vectors Upon Molecular Painting with the Complement Regulatory Protein CD59.Mol Biotechnol. 2016 Jul;58(7):480-8. doi: 10.1007/s12033-016-9944-z. Mol Biotechnol. 2016. PMID: 27170144 Free PMC article.
-
Dynamics and visualization of MCF7 adenocarcinoma cell death by aptamer-C1q-mediated membrane attack.Nucleic Acid Ther. 2012 Aug;22(4):275-82. doi: 10.1089/nat.2012.0355. Epub 2012 Aug 3. Nucleic Acid Ther. 2012. PMID: 22861487 Free PMC article.
-
COVID-19 Sepsis: Pathogenesis and Endothelial Molecular Mechanisms Based on "Two-Path Unifying Theory" of Hemostasis and Endotheliopathy-Associated Vascular Microthrombotic Disease, and Proposed Therapeutic Approach with Antimicrothrombotic Therapy.Vasc Health Risk Manag. 2021 Jun 1;17:273-298. doi: 10.2147/VHRM.S299357. eCollection 2021. Vasc Health Risk Manag. 2021. PMID: 34103921 Free PMC article. Review.
References
-
- Akatsu H, Yamada T, Okada N, Yamamoto T, Yamashina M, Okada H. Unique expression of HRF20 (CD59) in human nervous tissues. Microbiology and Immunology. 1997;41:321–329. - PubMed
-
- Bora NS, Gobleman CL, Atkinson JP, Pepose JS, Kapla H. Differential expression of the complement regulatory proteins in the human eye. Investigation in Ophthalmology and Visual Sciences. 1993;34:3579–3584. - PubMed
-
- Chang C, Husler T, Zhao J, Wiedmer T, Sims PJ. Identity of a peptide domain of human C9 that is bound by the cell-surface complement inhibitor, CD59. Journal of Biological Chemistry. 1994;269:26424–26430. - PubMed
-
- Discipio RG. Late components. In: Rother K, Till GO, Hansch GM, editors. The Complement System. 2. Berlin, Heidelberg: Springer Verlag; 1998. pp. 50–68.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

