Feedback inhibition in the inner plexiform layer underlies the surround-mediated responses of AII amacrine cells in the mammalian retina
- PMID: 11882691
- PMCID: PMC2290143
- DOI: 10.1113/jphysiol.2001.013133
Feedback inhibition in the inner plexiform layer underlies the surround-mediated responses of AII amacrine cells in the mammalian retina
Abstract
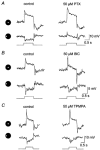
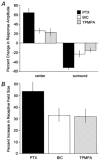
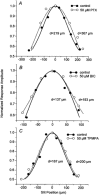
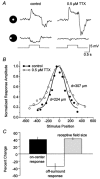
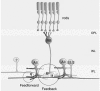
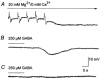
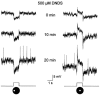
Intracellular recordings were made from narrow-field, bistratified AII amacrine cells in the isolated, superfused retina-eyecup of the rabbit. Pharmacological agents were applied to neurons to dissect the synaptic pathways subserving AII cells so as to determine the circuitry generating their off-surround responses. Application of the GABA antagonists, picrotoxin, bicuculline and 1,2,5,6-tetrahydropyridine-4-yl methylphosphinic acid (TPMPA) all increased the on-centre responses of AII amacrine cells, but attenuated the off-surround activity. At equal concentrations, picrotoxin was approximately twice as effective as bicuculline or TPMPA in modifying the response activity of AII amacrine cells. These results indicate that the mechanism underlying surround inhibition of AII amacrine cells includes activation of both GABA(A) and GABA(C) receptors in an approximately equal ratio. Application of the GABA antagonists also increased the size of on-centre receptive fields of AII amacrine cells. Again, picrotoxin was most effective, producing, on average, a 54 % increase in the size of the receptive field, whereas bicuculline and TPMPA produced comparable 34 and 33 % increases, respectfully. Application of the voltage-gated sodium channel blocker TTX produced effects on AII amacrine cells qualitatively similar to those of the GABA blockers. Intracellular application of the chloride channel blocker 4,4'-dinitro-stilbene-2,2'-disulphonic acid (DNDS) abolished the direct effects of GABA on AII amacrine cells. Moreover, DNDS increased the amplitude of both the on-centre and off-surround responses. The failure of DNDS to block the off-surround activity indicates that it is not mediated by direct GABAergic inhibition. Taken together, our results suggest that surround receptive fields of AII amacrine cells are generated indirectly by the GABAergic, reciprocal feedback synapses from S1/S2 amacrine cells to the axon terminals of rod bipolar cells.
Figures


Similar articles
-
Surround inhibition of mammalian AII amacrine cells is generated in the proximal retina.J Physiol. 2000 Mar 15;523 Pt 3(Pt 3):771-83. doi: 10.1111/j.1469-7793.2000.t01-1-00771.x. J Physiol. 2000. PMID: 10718754 Free PMC article.
-
I4AA-Sensitive chloride current contributes to the center light responses of bipolar cells in the tiger salamander retina.J Neurophysiol. 2000 Jun;83(6):3473-82. doi: 10.1152/jn.2000.83.6.3473. J Neurophysiol. 2000. PMID: 10848563
-
Modulation of excitatory synaptic transmission by GABA(C) receptor-mediated feedback in the mouse inner retina.J Neurophysiol. 2001 Nov;86(5):2285-98. doi: 10.1152/jn.2001.86.5.2285. J Neurophysiol. 2001. PMID: 11698519
-
Retinal bipolar cells receive negative feedback input from GABAergic amacrine cells.Vis Neurosci. 1988;1(3):297-305. doi: 10.1017/s0952523800001954. Vis Neurosci. 1988. PMID: 2856476 Review.
-
Electrical synapses between AII amacrine cells in the retina: Function and modulation.Brain Res. 2012 Dec 3;1487:160-72. doi: 10.1016/j.brainres.2012.05.060. Epub 2012 Jul 7. Brain Res. 2012. PMID: 22776293 Review.
Cited by
-
Dopamine and retinal function.Doc Ophthalmol. 2004 Jan;108(1):17-40. doi: 10.1023/b:doop.0000019487.88486.0a. Doc Ophthalmol. 2004. PMID: 15104164 Review.
-
Transience of the Retinal Output Is Determined by a Great Variety of Circuit Elements.Cells. 2022 Feb 25;11(5):810. doi: 10.3390/cells11050810. Cells. 2022. PMID: 35269432 Free PMC article. Review.
-
Masked excitatory crosstalk between the ON and OFF visual pathways in the mammalian retina.J Physiol. 2011 Sep 15;589(Pt 18):4473-89. doi: 10.1113/jphysiol.2011.213371. Epub 2011 Jul 18. J Physiol. 2011. PMID: 21768265 Free PMC article.
-
GABAA presynaptic inhibition regulates the gain and kinetics of retinal output neurons.Elife. 2021 Apr 27;10:e60994. doi: 10.7554/eLife.60994. Elife. 2021. PMID: 33904401 Free PMC article.
-
Non-canonical type 1 cannabinoid receptor signaling regulates night visual processing in the inner rat retina.iScience. 2024 May 7;27(6):109920. doi: 10.1016/j.isci.2024.109920. eCollection 2024 Jun 21. iScience. 2024. PMID: 38799553 Free PMC article.
References
-
- Bloomfield SA. Relationship between receptive and dendritic field size of amacrine cells in the rabbit retina. Journal of Neurophysiology. 1992;68:711–725. - PubMed
-
- Bloomfield SA. Effect of spike blockade on the receptive-field size of amacrine and ganglion cells in the rabbit retina. Journal of Neurophysiology. 1996;75:1878–1893. - PubMed
-
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Progress in Retina and Eye Research. 2001;20:351–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

