Electrical stimulation of the midbrain increases heart rate and arterial blood pressure in awake humans
- PMID: 11882692
- PMCID: PMC2290156
- DOI: 10.1113/jphysiol.2001.014621
Electrical stimulation of the midbrain increases heart rate and arterial blood pressure in awake humans
Abstract
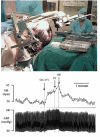
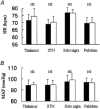
Electrical stimulation of the hypothalamus, basal ganglia or pedunculopontine nucleus in decorticate animals results in locomotion and a cardiorespiratory response resembling that seen during exercise. This has led to the hypothesis that parallel activation of cardiorespiratory and locomotor systems from the midbrain could form part of the 'central command' mechanism of exercise. However, the degree to which subcortical structures play a role in cardiovascular activation in awake humans has not been established. We studied the effects on heart rate (HR) and mean arterial blood pressure (MAP) of electrically stimulating the thalamus and basal ganglia in awake humans undergoing neurosurgery for movement disorders (n = 13 Parkinson's disease, n = 1 myoclonic dystonia, n = 1 spasmodic torticollis). HR and MAP increased during high frequency (> 90 Hz) electrical stimulation of the thalamus (HR 5 +/- 3 beats min(-1), P = 0.002, MAP 4 +/- 3 mmHg, P = 0.05, n = 9), subthalamic nucleus (HR 5 +/- 3 beats min(-1), P = 0.002, MAP 5 +/- 3 mmHg, P = 0.006, n = 8) or substantia nigra (HR 6 +/- 3 beats min(-1), P = 0.001, MAP 5 +/- 2 mmHg, P = 0.005, n = 8). This was accompanied by the facilitation of movement, but without the movement itself. Stimulation of the internal globus pallidus did not increase cardiovascular variables but did facilitate movement. Low frequency (< 20 Hz) stimulation of any site did not affect cardiovascular variables or movement. Electrical stimulation of the midbrain in awake humans can cause a modest increase in cardiovascular variables that is not dependent on movement feedback from exercising muscles. The relationship between this type of response and that occurring during actual exercise is unclear, but it indicates that subcortical command could be involved in 'parallel activation' of the locomotor and cardiovascular systems and thus contribute to the neurocircuitry of 'central command'.
Figures

Similar articles
-
Identifying cardiorespiratory neurocircuitry involved in central command during exercise in humans.J Physiol. 2007 Jan 15;578(Pt 2):605-12. doi: 10.1113/jphysiol.2006.122549. Epub 2006 Nov 2. J Physiol. 2007. PMID: 17082229 Free PMC article. Clinical Trial.
-
Regional (14C) 2-deoxyglucose uptake during vibrissae movements evoked by rat motor cortex stimulation.J Comp Neurol. 1982 Jul 1;208(3):255-87. doi: 10.1002/cne.902080305. J Comp Neurol. 1982. PMID: 7119161
-
Cardiovascular depressor responses to stimulation of substantia nigra and ventral tegmental area.Am J Physiol. 1997 Dec;273(6):H2549-57. doi: 10.1152/ajpheart.1997.273.6.H2549. Am J Physiol. 1997. PMID: 9435586
-
Chapter 33: the history of movement disorders.Handb Clin Neurol. 2010;95:501-46. doi: 10.1016/S0072-9752(08)02133-7. Handb Clin Neurol. 2010. PMID: 19892136 Review.
-
[Somatotopy in the basal ganglia].Brain Nerve. 2009 Dec;61(12):1383-94. Brain Nerve. 2009. PMID: 20034305 Review. Japanese.
Cited by
-
Infant locomotive development and its association with adult blood pressure.Eur J Pediatr. 2014 Oct;173(10):1309-17. doi: 10.1007/s00431-014-2326-2. Epub 2014 May 8. Eur J Pediatr. 2014. PMID: 24804637
-
Cardiac output-mediated regulation of cerebral blood flow during exercise: Clinical perspectives on the indirect impact of muscle metaboreflex.Exp Physiol. 2025 May;110(5):686-693. doi: 10.1113/EP091591. Epub 2024 Mar 18. Exp Physiol. 2025. PMID: 38500291 Free PMC article. Review.
-
Neurocardiology: translational advancements and potential.J Physiol. 2025 Mar;603(7):1729-1779. doi: 10.1113/JP284740. Epub 2024 Sep 27. J Physiol. 2025. PMID: 39340173 Free PMC article. Review.
-
Large-scale brainstem neuroimaging and genetic analyses provide new insights into the neuronal mechanisms of hypertension.HGG Adv. 2025 Jan 9;6(1):100392. doi: 10.1016/j.xhgg.2024.100392. Epub 2024 Dec 10. HGG Adv. 2025. PMID: 39663699 Free PMC article.
-
A brainstem monosynaptic excitatory pathway that drives locomotor activities and sympathetic cardiovascular responses.Nat Commun. 2022 Aug 29;13(1):5079. doi: 10.1038/s41467-022-32823-x. Nat Commun. 2022. PMID: 36038592 Free PMC article.
References
-
- Alexander E III, Kooy HM, van Herk M, Schwartz M, Barnes PD, Tarbell N, Mulkern RV, Holupka EJ, Loeffler JS. Magnetic resonance image-directed stereotactic neurosurgery: use of image fusion with computerized tomography to enhance spatial accuracy. Journal of Neurosurgery. 1995;83:271–276. - PubMed
-
- Angyan L. Cardiovascular effects of septal, thalamic, hypothalamic and midbrain self-stimulation. Physiology and Behaviour. 1978;20:217–226. - PubMed
-
- Angyan L. Substantia nigra stimulation and blood pressure effects of locally applied kainic acid. Neuroreport. 1991;2:785–788. - PubMed
-
- Angyan L. Somatomotor and cardiorespiratory responses to basal ganglia stimulation in cats. Physiology and Behaviour. 1994;56:167–173. - PubMed
-
- Angyan L. Cardiorespiratory effects of electrical stimulation of the globus pallidus in cats. Physiology and Behaviour. 1996;59:455–459. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

