Regions and activities of simian virus 40 T antigen that cooperate with an activated ras oncogene in transforming primary rat embryo fibroblasts
- PMID: 11884539
- PMCID: PMC136032
- DOI: 10.1128/jvi.76.7.3145-3157.2002
Regions and activities of simian virus 40 T antigen that cooperate with an activated ras oncogene in transforming primary rat embryo fibroblasts
Abstract
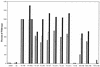
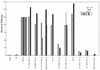
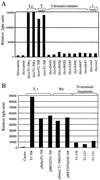
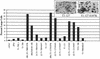
Prolonged expression of a ras oncogene in primary cells accelerates the natural process of senescence. This ras-induced permanent growth arrest is bypassed in cells expressing the simian virus 40 large T antigen. Previously we showed that two regions of T antigen, a region consisting of the N-terminal 147 amino acids and a region consisting of amino acids 251 to 708 (T251-708), independently overcome ras-induced senescence. Coexpression of either T-antigen fragment and Ras results in the appearance of dense foci of transformed cells. Using a series of mutants that produce shorter T-antigen fragments, we show that the C-terminal limit of the N-terminal T-antigen fragment that cooperates with Ras lies between amino acids 83 and 121. The N-terminal limit of the C-terminal T-antigen fragment lies between amino acids 252 and 271. In addition, we present evidence that cooperation between the N-terminal fragment and Ras depends upon an intact T-antigen J domain and the ability of the T antigen to bind and inactivate the growth-suppressive effect of the tumor suppressor Rb. Introduction of specific amino acid substitutions surrounding residue 400 into T251-708 prevented the fragment from cooperating with Ras. T251-708 proteins with these same substitutions inhibited the transcriptional transactivating potential of p53 as effectively as did the wild-type protein. Thus, at least one activity contained within T251-708, other than inactivating p53 as a transcriptional transactivator, is likely to be required to bypass Ras-induced senescence.
Figures



Similar articles
-
Simian virus 40 large T antigen contains two independent activities that cooperate with a ras oncogene to transform rat embryo fibroblasts.J Virol. 1995 Feb;69(2):923-34. doi: 10.1128/JVI.69.2.923-934.1995. J Virol. 1995. PMID: 7815561 Free PMC article.
-
Simian virus 40 large T antigen and two independent T-antigen segments sensitize cells to apoptosis following genotoxic damage.J Virol. 2002 Aug;76(16):8420-32. doi: 10.1128/jvi.76.16.8420-8432.2002. J Virol. 2002. PMID: 12134045 Free PMC article.
-
The amino-terminal functions of the simian virus 40 large T antigen are required to overcome wild-type p53-mediated growth arrest of cells.J Virol. 1994 Mar;68(3):1334-41. doi: 10.1128/JVI.68.3.1334-1341.1994. J Virol. 1994. PMID: 8107198 Free PMC article.
-
The p53 tumor suppressor gene and gene product.Princess Takamatsu Symp. 1989;20:221-30. Princess Takamatsu Symp. 1989. PMID: 2488233 Review.
-
p53 and transformation by SV40.Biol Cell. 1986;57(3):187-96. doi: 10.1111/j.1768-322x.1986.tb00475.x. Biol Cell. 1986. PMID: 3026531 Review.
Cited by
-
The role of protein tyrosine phosphatase 1B in Ras signaling.Proc Natl Acad Sci U S A. 2004 Feb 17;101(7):1834-9. doi: 10.1073/pnas.0304242101. Epub 2004 Feb 6. Proc Natl Acad Sci U S A. 2004. PMID: 14766979 Free PMC article.
-
Transcriptional control of SV40 T-antigen expression allows a complete reversion of immortalization.Nucleic Acids Res. 2004 Oct 14;32(18):5529-38. doi: 10.1093/nar/gkh887. Print 2004. Nucleic Acids Res. 2004. PMID: 15486202 Free PMC article.
-
Simian virus 40 T antigens and J domains: analysis of Hsp40 cochaperone functions in Escherichia coli.J Virol. 2003 Oct;77(19):10706-13. doi: 10.1128/jvi.77.19.10706-10713.2003. J Virol. 2003. PMID: 12970459 Free PMC article.
-
Cell-type specific regulation of gene expression by simian virus 40 T antigens.Virology. 2009 Mar 30;386(1):183-91. doi: 10.1016/j.virol.2008.12.038. Epub 2009 Feb 8. Virology. 2009. PMID: 19201438 Free PMC article.
-
Two independent regions of simian virus 40 T antigen increase CBP/p300 levels, alter patterns of cellular histone acetylation, and immortalize primary cells.J Virol. 2013 Dec;87(24):13499-509. doi: 10.1128/JVI.02658-13. Epub 2013 Oct 2. J Virol. 2013. PMID: 24089570 Free PMC article.
References
-
- Avantaggiati, M. L., V. Ogryzko, K. Gardner, A. Giordano, A. S. Levine, and K. Kelly. 1997. Recruitment of p300/CBP in p53-dependent signal pathways. Cell 89:1175-1184. - PubMed
-
- Barbacid, M. 1987. ras genes. Annu. Rev. Biochem. 56:779-827. - PubMed
-
- Campisi, J. 1997. The biology of replicative senescence. Eur. J. Cancer 33:703-709. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

