The M184V mutation reduces the selective excision of zidovudine 5'-monophosphate (AZTMP) by the reverse transcriptase of human immunodeficiency virus type 1
- PMID: 11884549
- PMCID: PMC136050
- DOI: 10.1128/jvi.76.7.3248-3256.2002
The M184V mutation reduces the selective excision of zidovudine 5'-monophosphate (AZTMP) by the reverse transcriptase of human immunodeficiency virus type 1
Abstract
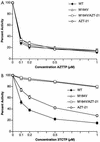
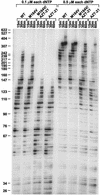
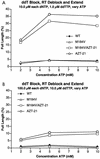
The M184V mutation in human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) causes resistance to lamivudine, but it also increases the sensitivity of the virus to zidovudine (3'-azido-3'-deoxythymidine; AZT). This sensitization to AZT is seen both in the presence and the absence of the mutations that confer resistance to AZT. AZT resistance is due to enhanced excision of AZT 5'-monophosphate (AZTMP) from the end of the primer by the RT of the resistant virus. Published data suggest that the excision reaction involves pyrophosphorolysis but that the likely in vivo pyrophosphate donor is not pyrophosphate but ATP. The mutations that lead to AZT resistance enhance ATP binding and, in so doing, enhance pyrophosphorolysis. The excision reaction is specific for AZT because HIV-1 RT, which can form a closed complex with a dideoxy-terminated primer and an incoming deoxynucleoside triphosphate (dNTP), does not form the closed complex with an AZTMP-terminated primer and an incoming dNTP. This means that an AZTMP-terminated primer has better access to the site where it can be excised. The M184V mutation alters the polymerase active site in a fashion that specifically interferes with ATP-mediated excision of AZTMP from the end of the primer strand. The M184V mutation does not affect the incorporation of AZT 5'-triphosphate (AZTTP), either in the presence or the absence of mutations that enhance AZTMP excision. However, in the presence of ATP, the M184V mutation does decrease the ability of HIV-1 RT to carry out AZTMP excision. Based on these results, and on the results of other excision experiments, we present a model to explain how the M184V mutation affects AZTMP excision.
Figures



Similar articles
-
Nucleoside analog resistance caused by insertions in the fingers of human immunodeficiency virus type 1 reverse transcriptase involves ATP-mediated excision.J Virol. 2002 Sep;76(18):9143-51. doi: 10.1128/jvi.76.18.9143-9151.2002. J Virol. 2002. PMID: 12186898 Free PMC article.
-
Selective excision of AZTMP by drug-resistant human immunodeficiency virus reverse transcriptase.J Virol. 2001 May;75(10):4832-42. doi: 10.1128/JVI.75.10.4832-4842.2001. J Virol. 2001. PMID: 11312355 Free PMC article.
-
Enhanced binding of azidothymidine-resistant human immunodeficiency virus 1 reverse transcriptase to the 3'-azido-3'-deoxythymidine 5'-monophosphate-terminated primer.J Biol Chem. 1998 Jun 5;273(23):14596-604. doi: 10.1074/jbc.273.23.14596. J Biol Chem. 1998. PMID: 9603976
-
Mutational patterns in the HIV genome and cross-resistance following nucleoside and nucleotide analogue drug exposure.Antivir Ther. 2001;6 Suppl 3:25-44. Antivir Ther. 2001. PMID: 11678471 Review.
-
Primer unblocking by HIV-1 reverse transcriptase and resistance to nucleoside RT inhibitors (NRTIs).Int J Biochem Cell Biol. 2004 Sep;36(9):1687-705. doi: 10.1016/j.biocel.2004.02.028. Int J Biochem Cell Biol. 2004. PMID: 15183338 Review.
Cited by
-
Selection of mutations in the connection and RNase H domains of human immunodeficiency virus type 1 reverse transcriptase that increase resistance to 3'-azido-3'-dideoxythymidine.J Virol. 2007 Aug;81(15):7852-9. doi: 10.1128/JVI.02203-06. Epub 2007 May 16. J Virol. 2007. PMID: 17507476 Free PMC article.
-
N348I in reverse transcriptase provides a genetic pathway for HIV-1 to select thymidine analogue mutations and mutations antagonistic to thymidine analogue mutations.AIDS. 2010 Mar 13;24(5):659-67. doi: 10.1097/QAD.0b013e328336781d. AIDS. 2010. PMID: 20160634 Free PMC article.
-
Two Coselected Distal Mutations in HIV-1 Reverse Transcriptase (RT) Alter Susceptibility to Nonnucleoside RT Inhibitors and Nucleoside Analogs.J Virol. 2019 May 15;93(11):e00224-19. doi: 10.1128/JVI.00224-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30894467 Free PMC article.
-
Mutations in the connection domain of HIV-1 reverse transcriptase increase 3'-azido-3'-deoxythymidine resistance.Proc Natl Acad Sci U S A. 2007 Jan 2;104(1):317-22. doi: 10.1073/pnas.0609642104. Epub 2006 Dec 19. Proc Natl Acad Sci U S A. 2007. PMID: 17179211 Free PMC article.
-
Crystal engineering of HIV-1 reverse transcriptase for structure-based drug design.Nucleic Acids Res. 2008 Sep;36(15):5083-92. doi: 10.1093/nar/gkn464. Epub 2008 Aug 1. Nucleic Acids Res. 2008. PMID: 18676450 Free PMC article.
References
-
- Arion, D., N. Kaushik, S. McCormick, G. Borkow, and M. A. Parniak. 1998. Phenotypic mechanism of HIV-1 resistance to 3′-azido-3′-deoxythymidine (AZT): increased polymerization processivity and enhanced sensitivity to pyrophosphate of the mutant viral reverse transcriptase. Biochemistry 37:15908-15917. - PubMed
-
- Back, N. K. T., M. Nijhuis, W. Keulen, C. A. B. Boucher, B. B. O. Essnik, A. B. P. van Kuilenburg, A. H. van Gennip, and B. Berkout. 1996. Reduced replication of 3TC-resistant HIV-1 variants in primary cells due to a processivity defect of the reverse transcriptase enzyme. EMBO J. 15:4040-4049. - PMC - PubMed
-
- Balzarini, J. 1999. Suppression of resistance to drugs targeted to human immunodeficiency virus reverse transcriptase by combination therapy. Biochem. Pharmacol. 58:1-27. - PubMed
-
- Boucher, C. A., N. Cammack, P. Schipper, R. Schurman, P. Rouse, M. A. Wainberg, and J. M. Cameron. 1993. High level resistance to (−) enantiomeric 2′-deoxy-3′-thiacytidine in vitro is due to one amino acid substitution in the catalytic site of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 37:2231-2234. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

