Herpes simplex virus tegument protein US11 interacts with conventional kinesin heavy chain
- PMID: 11884553
- PMCID: PMC136023
- DOI: 10.1128/jvi.76.7.3282-3291.2002
Herpes simplex virus tegument protein US11 interacts with conventional kinesin heavy chain
Abstract
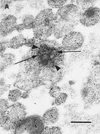
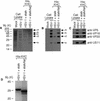
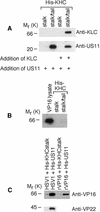
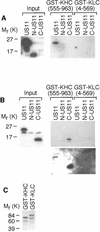
Little is known about the mechanisms of transport of neurotropic herpesviruses, such as herpes simplex virus (HSV), varicella-zoster virus, and pseudorabies virus, within neurons. For these viruses, which replicate in the nucleus, anterograde transport from the cell body of dorsal root ganglion (DRG) neurons to the axon terminus occurs over long distances. In the case of HSV, unenveloped nucleocapsids in human DRG neurons cocultured with autologous skin were observed by immunoelectron microscopy to colocalize with conventional ubiquitous kinesin, a microtubule-dependent motor protein, in the cell body and axon during anterograde axonal transport. Subsequently, four candidate kinesin-binding structural HSV proteins were identified (VP5, VP16, VP22, and US11) using oligohistidine-tagged human ubiquitous kinesin heavy chain (uKHC) as bait. Of these viral proteins, a direct interaction between uKHC and US11 was identified. In vitro studies identified residues 867 to 894 as the US11-binding site in uKHC located within the proposed heptad repeat cargo-binding domain of uKHC. In addition, the uKHC-binding site in US11 maps to the C-terminal RNA-binding domain. US11 is consistently cotransported with kinetics similar to those of the capsid protein VP5 into the axons of dissociated rat neurons, unlike the other tegument proteins VP16 and VP22. These observations suggest a major role for the uKHC-US11 interaction in anterograde transport of unenveloped HSV nucleocapsids in axons.
Figures




References
-
- Bloom, G. S., M. C. Wagner, K. K. Pfister, and S. T. Brady. 1988. Native structure and physical properties of bovine brain kinesin and identification of the ATP-binding subunit polypeptide. Biochemistry 27:3409-3416. - PubMed
-
- Cassady, K. A., M. Gross, and B. Roizman. 1998. The herpes simplex virus US11 protein effectively compensates for the γ134.5 gene if present before activation of protein kinase R by precluding its phosphorylation and that of the α subunit of eukaryotic translation initiation factor 2. J. Virol. 72:8620-8626. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

