Rotavirus NSP5: mapping phosphorylation sites and kinase activation and viroplasm localization domains
- PMID: 11884570
- PMCID: PMC136013
- DOI: 10.1128/jvi.76.7.3461-3470.2002
Rotavirus NSP5: mapping phosphorylation sites and kinase activation and viroplasm localization domains
Abstract
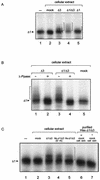
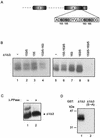
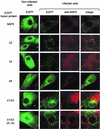
Rotavirus NSP5 is a nonstructural protein that localizes in cytoplasmic viroplasms of infected cells. NSP5 interacts with NSP2 and undergoes a complex posttranslational hyperphosphorylation, generating species with reduced polyacrylamide gel electrophoresis mobility. This process has been suggested to be due in part to autophosphorylation. We developed an in vitro phosphorylation assay using as a substrate an in vitro-translated NSP5 deletion mutant that was phosphorylated by extracts from MA104 cells transfected with NSP5 mutants but not by extracts from mock-transfected cells. The phosphorylated products obtained showed shifts in mobility similar to what occurs in vivo. From these and other experiments we concluded that NSP5 activates a cellular kinase(s) for its own phosphorylation. Three NSP5 regions were found to be essential for kinase(s) activation. Glutathione S-transferase-NSP5 mutants were produced in Escherichia coli and used to determine phosphoacceptor sites. These were mapped to four serines (Ser(153), Ser(155), Ser(163), and Ser(165)) within an acidic region with homology to casein kinase II (CKII) phosphorylation sites. CKII was able to phosphorylate NSP5 in vitro. NSP5 and its mutants fused to enhanced green fluorescent protein were used in transfection experiments followed by virus infection and allowed the determination of the domains essential for viroplasm localization in the context of virus infection.
Figures



Similar articles
-
Uncoupling substrate and activation functions of rotavirus NSP5: phosphorylation of Ser-67 by casein kinase 1 is essential for hyperphosphorylation.Proc Natl Acad Sci U S A. 2004 Nov 16;101(46):16304-9. doi: 10.1073/pnas.0406691101. Epub 2004 Nov 1. Proc Natl Acad Sci U S A. 2004. PMID: 15520389 Free PMC article.
-
Recombinant Rotaviruses Rescued by Reverse Genetics Reveal the Role of NSP5 Hyperphosphorylation in the Assembly of Viral Factories.J Virol. 2019 Dec 12;94(1):e01110-19. doi: 10.1128/JVI.01110-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31619556 Free PMC article.
-
A Genetically Engineered Rotavirus NSP2 Phosphorylation Mutant Impaired in Viroplasm Formation and Replication Shows an Early Interaction between vNSP2 and Cellular Lipid Droplets.J Virol. 2020 Jul 16;94(15):e00972-20. doi: 10.1128/JVI.00972-20. Print 2020 Jul 16. J Virol. 2020. PMID: 32461314 Free PMC article.
-
Plasmid-based reverse genetics for probing phosphorylation-dependent viroplasm formation in rotaviruses.Virus Res. 2021 Jan 2;291:198193. doi: 10.1016/j.virusres.2020.198193. Epub 2020 Oct 11. Virus Res. 2021. PMID: 33053412 Free PMC article. Review.
-
Viroplasms: Assembly and Functions of Rotavirus Replication Factories.Viruses. 2021 Jul 12;13(7):1349. doi: 10.3390/v13071349. Viruses. 2021. PMID: 34372555 Free PMC article. Review.
Cited by
-
The formation of viroplasm-like structures by the rotavirus NSP5 protein is calcium regulated and directed by a C-terminal helical domain.J Virol. 2007 Nov;81(21):11758-67. doi: 10.1128/JVI.01124-07. Epub 2007 Aug 15. J Virol. 2007. PMID: 17699573 Free PMC article.
-
MicroRNA-7 Inhibits Rotavirus Replication by Targeting Viral NSP5 In Vivo and In Vitro.Viruses. 2020 Feb 13;12(2):209. doi: 10.3390/v12020209. Viruses. 2020. PMID: 32069901 Free PMC article.
-
Rotavirus NSP2: A Master Orchestrator of Early Viral Particle Assembly.Viruses. 2024 May 21;16(6):814. doi: 10.3390/v16060814. Viruses. 2024. PMID: 38932107 Free PMC article. Review.
-
Iron‑sulfur clusters in viral proteins: Exploring their elusive nature, roles and new avenues for targeting infections.Biochim Biophys Acta Mol Cell Res. 2024 Jun;1871(5):119723. doi: 10.1016/j.bbamcr.2024.119723. Epub 2024 Apr 8. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 38599324 Free PMC article. Review.
-
Further characterisation of rotavirus cores: Ss(+)RNAs can be packaged in vitro but packaging lacks sequence specificity.Virus Res. 2013 Dec 26;178(2):252-63. doi: 10.1016/j.virusres.2013.09.034. Epub 2013 Oct 1. Virus Res. 2013. PMID: 24091366 Free PMC article.
References
-
- Afrikanova, I., E. Fabbretti, M. C. Miozzo, and O. R. Burrone. 1998. Rotavirus NSP5 phosphorylation is up-regulated by interaction with NSP2. J. Gen. Virol. 79:2679-2686. - PubMed
-
- Afrikanova, I., M. C. Miozzo, S. Giambiagi, and O. R. Burrone. 1996. Phosphorylation generates different forms of rotavirus NSP5. J. Gen. Virol. 77:2059-2065. - PubMed
-
- Ball, J. M., P. Tiang, C. Q.-Y. Zeng, A. P. Morris, and M. K. Estes. 1996. Age-depedent diarrhea induced by a rotaviral non-structural glycoprotein. Science 272:101-104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

