Structural analysis of the hepatitis C virus RNA polymerase in complex with ribonucleotides
- PMID: 11884572
- PMCID: PMC136026
- DOI: 10.1128/jvi.76.7.3482-3492.2002
Structural analysis of the hepatitis C virus RNA polymerase in complex with ribonucleotides
Abstract
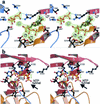
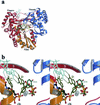
We report here the results of a systematic high-resolution X-ray crystallographic analysis of complexes of the hepatitis C virus (HCV) RNA polymerase with ribonucleoside triphosphates (rNTPs) and divalent metal ions. An unexpected observation revealed by this study is the existence of a specific rGTP binding site in a shallow pocket at the molecular surface of the enzyme, 30 A away from the catalytic site. This previously unidentified rGTP pocket, which lies at the interface between fingers and thumb, may be an allosteric regulatory site and could play a role in allowing alternative interactions between the two domains during a possible conformational change of the enzyme required for efficient initiation. The electron density map at 1.7-A resolution clearly shows the mode of binding of the guanosine moiety to the enzyme. In the catalytic site, density corresponding to the triphosphates of nucleotides bound to the catalytic metals was apparent in each complex with nucleotides. Moreover, a network of triphosphate densities was detected; these densities superpose to the corresponding moieties of the nucleotides observed in the initiation complex reported for the polymerase of bacteriophage phi6, strengthening the proposal that the two enzymes initiate replication de novo by similar mechanisms. No equivalent of the protein stacking platform observed for the priming nucleotide in the phi6 enzyme is present in HCV polymerase, however, again suggesting that a change in conformation of the thumb domain takes place upon template binding to allow for efficient de novo initiation of RNA synthesis.
Figures



References
-
- Ackermann, M., and R. Padmanabhan. 2001. De novo synthesis of RNA by the dengue virus RNA-dependent RNA polymerase exhibits temperature dependence at the initiation but not elongation phase. J. Biol. Chem. 276:39926-39937. - PubMed
-
- Ago, H., T. Adashi, A. Yoshida, M. Yamamoto, N. Habuka, K. Yatsunami, and M. Miyano. 1999. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Structure 7:1417-1426. - PubMed
-
- Arnold, J. J., and C. E. Cameron. 1999. Poliovirus RNA-dependent RNA polymerase (3Dpol) is sufficient for template switching in vitro. J. Biol. Chem. 274:2706-2716. - PubMed
-
- Arnold, J. J., S. K. Ghosh, and C. E. Cameron. 1999. Poliovirus RNA-dependent RNA polymerase (3D(pol)). Divalent cation modulation of primer, template, and nucleotide selection. J. Biol. Chem. 274:37060-37069. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

