Solid-phase proteoliposomes containing human immunodeficiency virus envelope glycoproteins
- PMID: 11884575
- PMCID: PMC136030
- DOI: 10.1128/jvi.76.7.3511-3521.2002
Solid-phase proteoliposomes containing human immunodeficiency virus envelope glycoproteins
Abstract
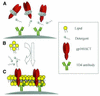
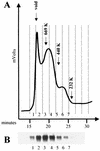
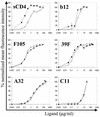
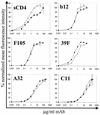
The human immunodeficiency virus type 1 (HIV-1) exterior envelope glycoprotein gp120 mediates receptor binding and is the major target for neutralizing antibodies. A broadly neutralizing antibody response is likely to be a critical component of the immune response against HIV-1. Although antibodies against monomeric gp120 are readily elicited in immunized individuals, these antibodies are inefficient in neutralizing primary HIV-1 isolates. As a chronic pathogen, HIV-1 has evolved to avoid an optimal host response by a number of immune escape mechanisms. Monomeric gp120 that has dissociated from the functional trimer presents irrelevant epitopes that are not accessible on functional trimeric envelope glycoproteins. The resulting low level of antigenic cross-reactivity between monomeric gp120 and the functional spike may contribute to the inability of monomeric gp120 to elicit broadly neutralizing antibodies. Attempts to generate native, trimeric envelope glycoproteins as immunogens have been frustrated by both the lability of the gp120-gp41 interaction and the weak association between gp120 subunits. Here, we present solid-phase HIV-1 gp160DeltaCT (cytoplasmic tail-deleted) proteoliposomes (PLs) containing native, trimeric envelope glycoproteins in a physiologic membrane setting. We present data that indicate that the gp160DeltaCT glycoproteins on PLs are trimers and are recognized by several relevant conformational ligands in a manner similar to that for gp160DeltaCT oligomers expressed on the cell surface. The PLs represent a significant advance over present envelope glycoprotein formulations as candidate immunogens for HIV vaccine design and development.
Figures




Similar articles
-
Selective recognition of oligomeric HIV-1 primary isolate envelope glycoproteins by potently neutralizing ligands requires efficient precursor cleavage.Virology. 2005 Feb 5;332(1):145-56. doi: 10.1016/j.virol.2004.10.042. Virology. 2005. PMID: 15661147
-
Analysis of the neutralizing antibody response elicited in rabbits by repeated inoculation with trimeric HIV-1 envelope glycoproteins.Virology. 2005 Jan 5;331(1):33-46. doi: 10.1016/j.virol.2004.09.022. Virology. 2005. PMID: 15582651
-
Characterization of the multiple conformational States of free monomeric and trimeric human immunodeficiency virus envelope glycoproteins after fixation by cross-linker.J Virol. 2006 Jul;80(14):6725-37. doi: 10.1128/JVI.00118-06. J Virol. 2006. PMID: 16809278 Free PMC article.
-
GP120: target for neutralizing HIV-1 antibodies.Annu Rev Immunol. 2006;24:739-69. doi: 10.1146/annurev.immunol.24.021605.090557. Annu Rev Immunol. 2006. PMID: 16551265 Review.
-
Challenges for structure-based HIV vaccine design.Curr Opin HIV AIDS. 2009 Sep;4(5):431-40. doi: 10.1097/COH.0b013e32832e6184. Curr Opin HIV AIDS. 2009. PMID: 20048708 Review.
Cited by
-
Neutralizing epitopes in the membrane-proximal external region of HIV-1 gp41 are influenced by the transmembrane domain and the plasma membrane.J Virol. 2012 Mar;86(6):2930-41. doi: 10.1128/JVI.06349-11. Epub 2012 Jan 11. J Virol. 2012. PMID: 22238313 Free PMC article.
-
Probing the Structure of the HIV-1 Envelope Trimer Using Aspartate Scanning Mutagenesis.J Virol. 2020 Oct 14;94(21):e01426-20. doi: 10.1128/JVI.01426-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32817217 Free PMC article.
-
Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies.J Virol. 2005 Aug;79(16):10108-25. doi: 10.1128/JVI.79.16.10108-10125.2005. J Virol. 2005. PMID: 16051804 Free PMC article.
-
Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates.Expert Rev Vaccines. 2006 Jun;5(3):347-63. doi: 10.1586/14760584.5.3.347. Expert Rev Vaccines. 2006. PMID: 16827619 Free PMC article. Review.
-
Hyperglycosylated mutants of human immunodeficiency virus (HIV) type 1 monomeric gp120 as novel antigens for HIV vaccine design.J Virol. 2003 May;77(10):5889-901. doi: 10.1128/jvi.77.10.5889-5901.2003. J Virol. 2003. PMID: 12719582 Free PMC article.
References
-
- Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. - PubMed
-
- Babcock, G. J., T. Mirzabekov, W. Wojtowicz, and J. Sodroski. 2001. Ligand-binding characteristics of CXCR4 incorporated into paramagnetic proteoliposomes. J. Biol. Chem. 276:38433-38440. - PubMed
-
- Berman, P. W., T. J. Gregory, L. Riddle, G. R. Nakamura, M. A. Champe, J. P. Porter, F. M. Wurm, R. D. Hershberg, E. K. Cobb, and J. W. Eichberg. 1990. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature 345:622-625. - PubMed
-
- Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. - PMC - PubMed
-
- Bruck, C., C. Thiriart, L. Fabry, M. Francotte, P. Pala, O. Van Opstal, J. Culp, M. Rosenberg, M. De Wilde, P. Heidt, et al. 1994. HIV-1 envelope-elicited neutralizing antibody titres correlate with protection and virus load in chimpanzees. Vaccine 12:1141-1148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

