Capsid protein C of tick-borne encephalitis virus tolerates large internal deletions and is a favorable target for attenuation of virulence
- PMID: 11884577
- PMCID: PMC136049
- DOI: 10.1128/jvi.76.7.3534-3543.2002
Capsid protein C of tick-borne encephalitis virus tolerates large internal deletions and is a favorable target for attenuation of virulence
Abstract
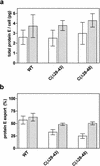
Deletions ranging in size from 4 to 21 amino acid residues were introduced into the capsid protein of the flavivirus tick-borne encephalitis (TBE) virus. These deletions incrementally affected a hydrophobic domain which is present at the center of all flavivirus capsid protein sequences and part of which may form an amphipathic alpha-helix. In the context of the full-length TBE genome, the deletions did not measurably affect protein expression and up to a deletion length of 16 amino acid residues, corresponding to almost 17% of mature protein C, viable virus was recovered. This virus was strongly attenuated but highly immunogenic in adult mice, revealing capsid protein C as a new and attractive target for the directed attenuation of flaviviruses. Apparently, the larger deletions interfered with the correct assembly of infectious virus particles, and this disturbance of virion assembly is likely to be the molecular basis of attenuation. However, all of the mutants carrying large deletions produced substantial amounts of subviral particles, which as judged from density gradient analyses were identical to recombinant subviral particles as obtained by the expression of the surface proteins prM and E alone. The structural and functional flexibility of protein C revealed in this study and its predicted largely alpha-helical conformation are reminiscent of capsid proteins of other enveloped viruses, such as alphaviruses (N-terminal domain of the capsid protein), retroviruses, and hepadnaviruses and suggest that all of these may belong to a common structural class, which is fundamentally distinct from the classical beta-barrel structures of many icosahedral viral capsids. The possibility of attenuating flaviviruses by disturbing virus assembly and favoring the production of noninfectious but highly immunogenic subviral particles opens up a promising new avenue for the development of live flavivirus vaccines.
Figures



Similar articles
-
Infectious cDNA clone of attenuated Langat tick-borne flavivirus (strain E5) and a 3' deletion mutant constructed from it exhibit decreased neuroinvasiveness in immunodeficient mice.Virology. 2001 Apr 10;282(2):288-300. doi: 10.1006/viro.2001.0846. Virology. 2001. PMID: 11289811
-
Synergistic Internal Ribosome Entry Site/MicroRNA-Based Approach for Flavivirus Attenuation and Live Vaccine Development.mBio. 2017 Apr 18;8(2):e02326-16. doi: 10.1128/mBio.02326-16. mBio. 2017. PMID: 28420742 Free PMC article.
-
Spontaneous and engineered deletions in the 3' noncoding region of tick-borne encephalitis virus: construction of highly attenuated mutants of a flavivirus.J Virol. 1998 Mar;72(3):2132-40. doi: 10.1128/JVI.72.3.2132-2140.1998. J Virol. 1998. PMID: 9499069 Free PMC article.
-
Chimeric flaviviruses: novel vaccines against dengue fever, tick-borne encephalitis, and Japanese encephalitis.Adv Virus Res. 2003;61:469-509. doi: 10.1016/s0065-3527(03)61013-4. Adv Virus Res. 2003. PMID: 14714441 Review.
-
Flavivirus immunization with capsid-deletion mutants: basics, benefits, and barriers.Viral Immunol. 2004;17(4):461-72. doi: 10.1089/vim.2004.17.461. Viral Immunol. 2004. PMID: 15671744 Review.
Cited by
-
Isolation of capsid protein dimers from the tick-borne encephalitis flavivirus and in vitro assembly of capsid-like particles.J Virol. 2004 Aug;78(15):8078-84. doi: 10.1128/JVI.78.15.8078-8084.2004. J Virol. 2004. PMID: 15254179 Free PMC article.
-
Core protein-mediated 5'-3' annealing of the West Nile virus genomic RNA in vitro.Virus Res. 2012 Aug;167(2):226-35. doi: 10.1016/j.virusres.2012.05.003. Epub 2012 May 28. Virus Res. 2012. PMID: 22652509 Free PMC article.
-
Dengue virus capsid protein usurps lipid droplets for viral particle formation.PLoS Pathog. 2009 Oct;5(10):e1000632. doi: 10.1371/journal.ppat.1000632. Epub 2009 Oct 23. PLoS Pathog. 2009. PMID: 19851456 Free PMC article.
-
A heterologous coiled coil can substitute for helix I of the Sindbis virus capsid protein.J Virol. 2003 Aug;77(15):8345-53. doi: 10.1128/jvi.77.15.8345-8353.2003. J Virol. 2003. PMID: 12857904 Free PMC article.
-
Tick-borne Encephalitis Vaccines.J Bioterror Biodef. 2011;2011(Suppl 1):3. doi: 10.4172/2157-2526.S1-003. J Bioterror Biodef. 2011. PMID: 23997980 Free PMC article.
References
-
- Bamford, J. K., D. H. Bamford, T. Li, and G. J. Thomas, Jr. 1993. Structural studies of the enveloped dsRNA bacteriophage phi 6 of Pseudomonas syringae by Raman spectroscopy. II. Nucleocapsid structure and thermostability of the virion, nucleocapsid and polymerase complex. J. Mol. Biol. 230:473-482. - PubMed
-
- Burke, D. S., and T. P. Monath. 2001. Flaviviruses, p. 1043-1125. In D. M. Knipe, P. M. Howley, et al. (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
-
- Choi, H. K., G. Lu, S. Lee, G. Wengler, and M. G. Rossmann. 1997. The structure of Semliki Forest virus core protein. Proteins Struct. Funct. Genet. 27:345-359. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

