Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors
- PMID: 11884591
- PMCID: PMC133681
- DOI: 10.1128/MCB.22.7.2025-2036.2002
Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors
Abstract
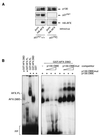
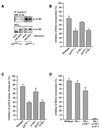
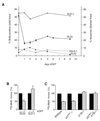
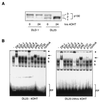
AFX-like Forkhead transcription factors, which are controlled by phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, are involved in regulating cell cycle progression and cell death. Both cell cycle arrest and induction of apoptosis are mediated in part by transcriptional regulation of p27(kip1). Here we show that the Forkheads AFX (FOXO4) and FKHR-L1 (FOXO3a) also directly control transcription of the retinoblastoma-like p130 protein and cause upregulation of p130 protein expression. Detailed analysis of p130 regulation demonstrates that following Forkhead-induced cell cycle arrest, cells enter G(0) and become quiescent. This is shown by a change in phosphorylation of p130 to G(0)-specific forms and increased p130/E2F-4 complex formation. Most importantly, long-term Forkhead activation causes a sustained but reversible inhibition of proliferation without a marked increase in apoptosis. As for the activity of the Forkheads, we also show that protein levels of p130 are controlled by endogenous PI3K/PKB signaling upon cell cycle reentry. Surprisingly, not only nontransformed cells, but also cancer cells such as human colon carcinoma cells, are forced into quiescence by Forkhead activation. We therefore propose that Forkhead inactivation by PKB signaling in quiescent cells is a crucial step in cell cycle reentry and contributes to the processes of transformation and regeneration.
Figures





References
-
- Albrecht, J. H., R. Y. Poon, C. L. Ahonen, B. M. Rieland, C. Deng, and G. S. Crary. 1998. Involvement of p21 and p27 in the regulation of CDK activity and cell cycle progression in the regenerating liver. Oncogene 16:2141-2150. - PubMed
-
- Baldi, A., V. Esposito, A. De Luca, C. M. Howard, G. Mazzarella, F. Baldi, M. Caputi, and A. Giordano. 1996. Differential expression of the retinoblastoma gene family members pRb/p105, p107, and pRb2/p130 in lung cancer. Clin. Cancer Res. 2:1239-1245. - PubMed
-
- Bos, J. L. 1995. p21ras: an oncoprotein functioning in growth factor-induced signal transduction. Eur. J. Cancer 31:1051-1054. - PubMed
-
- Bouzahzah, B., M. Fu, A. Iavarone, V. M. Factor, S. S. Thorgeirsson, and R. G. Pestell. 2000. Transforming growth factor-β1 recruits histone deacetylase 1 to a p130 repressor complex in transgenic mice in vivo. Cancer Res. 60:4531-4537. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
