Functional divergence between histone deacetylases in fission yeast by distinct cellular localization and in vivo specificity
- PMID: 11884604
- PMCID: PMC133699
- DOI: 10.1128/MCB.22.7.2170-2181.2002
Functional divergence between histone deacetylases in fission yeast by distinct cellular localization and in vivo specificity
Erratum in
- Mol Cell Biol 2002 Jul;22(14):5257-8
Abstract
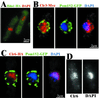
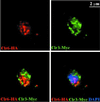
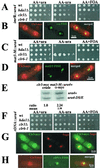
Histone deacetylases (HDACs) are important for gene regulation and the maintenance of heterochromatin in eukaryotes. Schizosaccharomyces pombe was used as a model system to investigate the functional divergence within this conserved enzyme family. S. pombe has three HDACs encoded by the hda1(+), clr3(+), and clr6(+) genes. Strains mutated in these genes have previously been shown to display strikingly different phenotypes when assayed for viability, chromosome loss, and silencing. Here, conserved differences in the substrate binding pocket identify Clr6 and Hda1 as class I HDACs, while Clr3 belongs in the class II family. Furthermore, these HDACs were shown to have strikingly different subcellular localization patterns. Hda1 was localized to the cytoplasm, while most of Clr3 resided throughout the nucleus. Finally, Clr6 was localized exclusively on the chromosomes in a spotted pattern. Interestingly, Clr3, the only HDAC present in the nucleolus, was required for ribosomal DNA (rDNA) silencing. Clr3 presumably acts directly on heterochromatin, since it colocalized with the centromere, mating-type region, and rDNA as visualized by in situ hybridization. In addition, Clr3 could be cross-linked to mat3 in chromatin immunoprecipitation experiments. Western analysis of bulk histone preparations indicated that Hda1 (class I) had a generally low level of activity in vivo and Clr6 (class I) had a high level of activity and broad in vivo substrate specificity, whereas Clr3 (class II) displayed its main activity on acetylated lysine 14 of histone H3. Thus, the distinct functions of the S. pombe HDACs are likely explained by their distinct cellular localization and their different in vivo specificities.
Figures




References
-
- Allshire, R. C., E. R. Nimmo, K. Ekwall, J. P. Javerzat, and G. Cranston. 1995. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 9:218-233. - PubMed
-
- Bahler, J., J. Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie III, A. B. Steever, A. Wach, P. Philippsen, and J. R. Pringle. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14:943-951. - PubMed
-
- Bilsland, E., M. Dahlen, and P. Sunnerhagen. 1998. Genomic disruption of six budding yeast genes gives one drastic example of phenotype strain-dependence. Yeast 14:655-664. - PubMed
-
- Brownell, J. E., J. Zhou, T. Ranalli, R. Kobayashi, D. G. Edmondson, S. Y. Roth, and C. D. Allis. 1996. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 84:843-851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
