The ubiquitin ligase component Siah1a is required for completion of meiosis I in male mice
- PMID: 11884614
- PMCID: PMC133675
- DOI: 10.1128/MCB.22.7.2294-2303.2002
The ubiquitin ligase component Siah1a is required for completion of meiosis I in male mice
Abstract
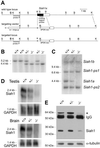
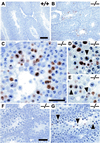
The mammalian Siah genes encode highly conserved proteins containing a RING domain. As components of E3 ubiquitin ligase complexes, Siah proteins facilitate the ubiquitination and degradation of diverse protein partners including beta-catenin, N-CoR, and DCC. We used gene targeting in mice to analyze the function of Siah1a during mammalian development and reveal novel roles in growth, viability, and fertility. Mutant animals have normal weights at term but are postnatally growth retarded, despite normal levels of pituitary growth hormone. Embryonic fibroblasts isolated from mutant animals grow normally. Most animals die before weaning, and few survive beyond 3 months. Serum gonadotropin levels are normal in Siah1a mutant mice; however, females are subfertile and males are sterile due to a block in spermatogenesis. Although spermatocytes in mutant mice display normal meiotic prophase and meiosis I spindle formation, they accumulate at metaphase to telophase of meiosis I and subsequently undergo apoptosis. The requirement of Siah1a for normal progression beyond metaphase I suggests that Siah1a may be part of a novel E3 complex acting late in the first meiotic division.
Figures




References
-
- Amson, R. B., M. Nemani, J. P. Roperch, D. Israeli, L. Bougueleret, I. Le Gall, M. Medhioub, G. Linares-Cruz, F. Lethrosne, P. Pasturaud, L. Piouffre, S. Prieur, et al. 1996. Isolation of 10 differentially expressed cDNAs in p53-induced apoptosis: activation of the vertebrate homologue of the Drosophila seven in absentia gene. Proc. Natl. Acad. Sci. USA 93:3953-3957. - PMC - PubMed
-
- Antonio, C., I. Ferby, H. Wilhelm, M. Jones, E. Karsenti, A. R. Nebreda, and I. Vernos. 2000. Xkid, a chromokinesin required for chromosome alignment on the metaphase plate. Cell 102:425-435. - PubMed
-
- Baker, S. M., A. W. Plug, T. A. Prolla, C. E. Bronner, A. C. Harris, X. Yao, D. M. Christie, C. Monell, N. Arnheim, A. Bradley, T. Ashley, and R. M. Liskay. 1996. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat. Genet. 13:336-342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
