Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae
- PMID: 11884617
- PMCID: PMC133690
- DOI: 10.1128/MCB.22.7.2329-2344.2002
Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae
Abstract
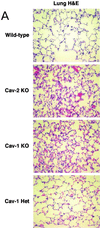
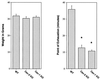
Caveolin-2 is a member of the caveolin gene family with no known function. Although caveolin-2 is coexpressed and heterooligomerizes with caveolin-1 in many cell types (most notably adipocytes and endothelial cells), caveolin-2 has traditionally been considered the dispensable structural partner of the widely studied caveolin-1. We now directly address the functional significance of caveolin-2 by genetically targeting the caveolin-2 locus (Cav-2) in mice. In the absence of caveolin-2 protein expression, caveolae still form and caveolin-1 maintains its localization in plasma membrane caveolae, although in certain tissues caveolin-1 is partially destabilized and shows modestly diminished protein levels. Despite an intact caveolar membrane system, the Cav-2-null lung parenchyma shows hypercellularity, with thickened alveolar septa and an increase in the number of endothelial cells. As a result of these pathological changes, these Cav-2-null mice are markedly exercise intolerant. Interestingly, these Cav-2-null phenotypes are identical to the ones we and others have recently reported for Cav-1-null mice. As caveolin-2 expression is also severely reduced in Cav-1-null mice, we conclude that caveolin-2 deficiency is the clear culprit in this lung disorder. Our analysis of several different phenotypes observed in caveolin-1-deficient mice (i.e., abnormal vascular responses and altered lipid homeostasis) reveals that Cav-2-null mice do not show any of these other phenotypes, indicating a selective role for caveolin-2 in lung function. Taken together, our data show for the first time a specific role for caveolin-2 in mammalian physiology independent of caveolin-1.
Figures














Similar articles
-
Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities.J Biol Chem. 2001 Oct 12;276(41):38121-38. doi: 10.1074/jbc.M105408200. Epub 2001 Jul 16. J Biol Chem. 2001. PMID: 11457855
-
Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities.J Biol Chem. 2002 Mar 8;277(10):8635-47. doi: 10.1074/jbc.M110970200. Epub 2001 Dec 5. J Biol Chem. 2002. PMID: 11739396
-
Caveolin-1 expression is critical for vascular endothelial growth factor-induced ischemic hindlimb collateralization and nitric oxide-mediated angiogenesis.Circ Res. 2004 Jul 23;95(2):154-61. doi: 10.1161/01.RES.0000136344.27825.72. Epub 2004 Jun 17. Circ Res. 2004. PMID: 15205364
-
The biology of caveolae: lessons from caveolin knockout mice and implications for human disease.Mol Interv. 2003 Dec;3(8):445-64. doi: 10.1124/mi.3.8.445. Mol Interv. 2003. PMID: 14993453 Review.
-
Getting rid of caveolins: phenotypes of caveolin-deficient animals.Biochim Biophys Acta. 2005 Dec 30;1746(3):322-33. doi: 10.1016/j.bbamcr.2005.06.001. Epub 2005 Jun 23. Biochim Biophys Acta. 2005. PMID: 16019085 Review.
Cited by
-
Homotrimer cavin1 interacts with caveolin1 to facilitate tumor growth and activate microglia through extracellular vesicles in glioma.Theranostics. 2020 May 17;10(15):6674-6694. doi: 10.7150/thno.45688. eCollection 2020. Theranostics. 2020. PMID: 32550897 Free PMC article.
-
MMTV promoter-regulated caveolin-1 overexpression yields defective parenchymal epithelia in multiple exocrine organs of transgenic mice.Exp Mol Pathol. 2010 Aug;89(1):9-19. doi: 10.1016/j.yexmp.2010.03.009. Epub 2010 Apr 22. Exp Mol Pathol. 2010. PMID: 20399205 Free PMC article.
-
The early nutritional environment of mice determines the capacity for adipose tissue expansion by modulating genes of caveolae structure.PLoS One. 2010 Jun 21;5(6):e11015. doi: 10.1371/journal.pone.0011015. PLoS One. 2010. PMID: 20574519 Free PMC article.
-
Caveolin-1 and MLRs: A potential target for neuronal growth and neuroplasticity after ischemic stroke.Int J Med Sci. 2019 Oct 15;16(11):1492-1503. doi: 10.7150/ijms.35158. eCollection 2019. Int J Med Sci. 2019. PMID: 31673241 Free PMC article. Review.
-
Tyrosine phosphorylation-dependence of caveolae-mediated endocytosis.J Cell Mol Med. 2007 Nov-Dec;11(6):1239-50. doi: 10.1111/j.1582-4934.2007.00127.x. J Cell Mol Med. 2007. PMID: 18205698 Free PMC article. Review.
References
-
- Crouch, E. 1990. Pathobiology of pulmonary fibrosis. Am. J. Physiol. 259:L159-184. - PubMed
-
- Das, K., R. Y. Lewis, P. E. Scherer, and M. P. Lisanti. 1999. The membrane spanning domains of caveolins 1 and 2 mediate the formation of caveolin heterooligomers: implications for the assembly of caveolae membranes in vivo. J. Biol. Chem. 274:18721-18726. - PubMed
-
- Dormans, J. A. 1983. The ultrastructure of various cell types in the lung of the rat: a survey. Exp. Pathol. 24:15-33. - PubMed
-
- Drab, M., P. Verkade, M. Elger, M. Kasper, M. Lohn, B. Lauterbach, J. Menne, C. Lindschau, F. Mende, F. C. Luft, A. Schedl, H. Haller, and T. V. Kurzchalia. 2001. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293:2449-2452. - PubMed
-
- Edelmann, W., K. Yang, A. Umar, J. Heyer, K. Lau, K. Fan, W. Liedtke, P. E. Cohen, M. F. Kane, J. R. Lipford, N. Yu, G. F. Crouse, J. W. Pollard, T. Kunkel, M. Lipkin, R. Kolodner, and R. Kucherlapati. 1997. Mutation in the mismatch repair gene Msh6 causes cancer susceptibility. Cell 91:467-477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
