Protein phosphatase 2A forms a molecular complex with Shc and regulates Shc tyrosine phosphorylation and downstream mitogenic signaling
- PMID: 11884620
- PMCID: PMC133677
- DOI: 10.1128/MCB.22.7.2375-2387.2002
Protein phosphatase 2A forms a molecular complex with Shc and regulates Shc tyrosine phosphorylation and downstream mitogenic signaling
Abstract
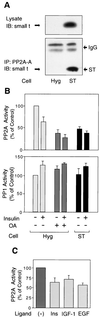
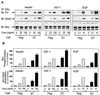
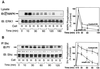
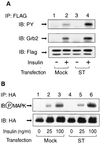
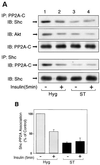
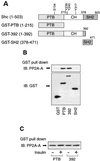
Protein phosphatase 2A (PP2A) is a multimeric serine/threonine phosphatase that carries out multiple functions. Although numerous observations suggest that PP2A plays a major role in downregulation of the mitogen-activated protein (MAP) kinase pathway, the precise mechanisms are unknown. To clarify the role of PP2A in growth factor (insulin, epidermal growth factor [EGF], and insulin-like growth factor 1 [IGF-1]) stimulation of the Ras/MAP kinase pathway, simian virus 40 small t antigen was expressed in Rat-1 fibroblasts which overexpress insulin receptors. Small t antigen is known to specifically inhibit PP2A by binding to the A PP2A regulatory subunit, interfering with the ability of PP2A to bind to its cellular substrates. Overexpressed small t protein was coimmunoprecipitated with PP2A and inhibited cellular PP2A activity but did not inhibit protein phosphatase 1 (PP1) activity. Insulin, IGF-1, and EGF stimulation also inhibited PP2A activity. Growth factor-stimulated Ras, Raf-1, MAP kinase, and mitogen-activated extracellular-signal-regulated kinase kinase (MEK) activities were elevated in small-t-antigen-expressing cells. Furthermore, Shc tyrosine phosphorylation and its association with Grb2 were also elevated in small-t-antigen-expressing cells. Expression levels of Shc, Ras, MEK, or MAP kinase and phosphorylation of insulin, EGF, and IGF-1 receptors were not altered. Interestingly, we found that PP2A associated with Shc in the basal state and dissociated in response to insulin and EGF and that this dissociation was inhibited by 65% in small-t-antigen-expressing cells. In addition, we found that PP2A associates with the phosphotyrosine-binding domain (PTB domain) of Shc and that phosphorylation of tyrosine 317 of Shc was required for PP2A-Shc dissociation. We conclude (i) that PP2A negatively regulates the Ras/MAP kinase pathway by binding to Shc, inhibiting tyrosine phosphorylation; (ii) that the Shc-PP2A association is mediated by the Shc PTB domain but the interaction is independent of phosphotyrosine binding, indicating a new molecular function for the PTB domain; (iii) that growth factor stimulation, or small-t-antigen expression, causes dissociation of the PP2A-Shc complex, facilitating Shc phosphorylation and downstream activations of the Ras/MAP kinase pathway; and (iv) that this defines a new mechanism of small-t-antigen action to promote mitogenesis.
Figures







Similar articles
-
Protein phosphatase 2A negatively regulates insulin's metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes.Mol Cell Biol. 2004 Oct;24(19):8778-89. doi: 10.1128/MCB.24.19.8778-8789.2004. Mol Cell Biol. 2004. PMID: 15367694 Free PMC article.
-
Roles of insulin receptor substrate-1 and Shc on insulin-like growth factor I receptor signaling in early passages of cultured human fibroblasts.Endocrinology. 1997 Feb;138(2):741-50. doi: 10.1210/endo.138.2.4910. Endocrinology. 1997. PMID: 9003010
-
Increased insulin sensitivity in IGF-I receptor--deficient brown adipocytes.Diabetes. 2002 Mar;51(3):743-54. doi: 10.2337/diabetes.51.3.743. Diabetes. 2002. PMID: 11872675
-
STRIPAK complexes: structure, biological function, and involvement in human diseases.Int J Biochem Cell Biol. 2014 Feb;47:118-48. doi: 10.1016/j.biocel.2013.11.021. Epub 2013 Dec 11. Int J Biochem Cell Biol. 2014. PMID: 24333164 Free PMC article. Review.
-
Lymphocyte antigen receptor signal integration and regulation by the SHC adaptor.Biol Chem. 1999 Feb;380(2):129-34. doi: 10.1515/BC.1999.020. Biol Chem. 1999. PMID: 10195419 Review.
Cited by
-
NNMT Silencing Activates Tumor Suppressor PP2A, Inactivates Oncogenic STKs, and Inhibits Tumor Forming Ability.Clin Cancer Res. 2017 May 1;23(9):2325-2334. doi: 10.1158/1078-0432.CCR-16-1323. Epub 2016 Nov 3. Clin Cancer Res. 2017. PMID: 27810903 Free PMC article.
-
LB100, a small molecule inhibitor of PP2A with potent chemo- and radio-sensitizing potential.Cancer Biol Ther. 2015;16(6):821-33. doi: 10.1080/15384047.2015.1040961. Epub 2015 Apr 21. Cancer Biol Ther. 2015. PMID: 25897893 Free PMC article. Review.
-
Molecular dynamics simulations reveal that Tyr-317 phosphorylation reduces Shc binding affinity for phosphotyrosyl residues of epidermal growth factor receptor.Biophys J. 2009 Mar 18;96(6):2278-88. doi: 10.1016/j.bpj.2008.11.018. Biophys J. 2009. PMID: 19289054 Free PMC article.
-
Phenothiazines induce PP2A-mediated apoptosis in T cell acute lymphoblastic leukemia.J Clin Invest. 2014 Feb;124(2):644-55. doi: 10.1172/JCI65093. Epub 2014 Jan 9. J Clin Invest. 2014. PMID: 24401270 Free PMC article.
-
Signal transduction therapeutics: relevance for Alzheimer's disease.J Mol Neurosci. 2004;23(1-2):123-42. doi: 10.1385/JMN:23:1-2:123. J Mol Neurosci. 2004. PMID: 15126698 Review.
References
-
- Abraham, D., K. Podar, M. Pacher, M. Kubicek, N. Welzel, B. A. Hemmings, S. M. Dilworth, H. Mischak, W. Kolch, and M. Baccarini. 2000. Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J. Biol. Chem. 275:22300-22304. - PubMed
-
- Alessi, D. R., N. Gomez, G. Moorhead, T. Lewis, S. M. Keyse, and P. Cohen. 1995. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr. Biol. 5:283-295. - PubMed
-
- Anderson, N. G., J. L. Maller, N. K. Tonks, and T. W. Sturgill. 1990. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature 343:651-653. - PubMed
-
- Andjelkovic, M., T. Jakubowicz, P. Cron, X. F. Ming, J. W. Han, and B. A. Hemmings. 1996. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc. Natl. Acad. Sci. USA 93:5699-5704. - PMC - PubMed
-
- Begum, N., and L. Ragolia. 1996. cAMP counter-regulates insulin-mediated protein phosphatase-2A inactivation in rat skeletal muscle cells. J. Biol. Chem. 271:31166-31171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
