Boron-containing aptamers to ATP
- PMID: 11884639
- PMCID: PMC101341
- DOI: 10.1093/nar/30.6.1401
Boron-containing aptamers to ATP
Abstract
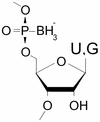
Boron neutron capture therapy (BNCT), an experimental treatment for certain cancers, destroys only cells near the boron; however, there is a need to develop highly specific delivery agents. As nucleic acid aptamers recognize specific molecular targets, we investigated the influence of boronated nucleotide analogs on RNA function and on the systematic evolution of ligands by exponential enrichment (SELEX) process. Substitution of guanosine 5'-(alpha-P-borano) triphosphate (bG) for GTP or uridine 5'-(alpha-P-borano) triphosphate (bU) for UTP in several known aptamers diminished or eliminated target recognition by those RNAs. Specifically, ATP-binding aptamers containing the zeta-fold, which appears in several selections for adenosine aptamers, became inactive upon bG substitution but were only moderately affected by bU substitution. Selections were carried out using the bG or bU analogs with C8-linked ATP agarose as the binding target. The selections with bU and normal NTP yielded some zeta-fold aptamers, while the bG selection yielded none of this type. Non-zeta aptamers from bU and bG populations tolerated the borano substitution and many required it. The borano nucleotide requirement is specific; bU could not be used in bG-dependent aptamers nor vice versa. The borano group plays an essential role, as yet undefined, in target recognition or RNA structure. We conclude that the bG and bU nucleotides are fully compatible with SELEX, and that these analogs could be used to make boronated aptamers as therapeutics for BNCT.
Figures



Similar articles
-
New NTP analogs: the synthesis of 4'-thioUTP and 4'-thioCTP and their utility for SELEX.Nucleic Acids Res. 2005 May 24;33(9):2942-51. doi: 10.1093/nar/gki578. Print 2005. Nucleic Acids Res. 2005. PMID: 15914669 Free PMC article.
-
Selection and evolution of NTP-specific aptamers.Nucleic Acids Res. 2004 Sep 27;32(17):5045-58. doi: 10.1093/nar/gkh835. Print 2004. Nucleic Acids Res. 2004. PMID: 15452272 Free PMC article.
-
RNA aptamers to the adenosine moiety of S-adenosyl methionine: structural inferences from variations on a theme and the reproducibility of SELEX.Nucleic Acids Res. 1997 May 15;25(10):2020-4. doi: 10.1093/nar/25.10.2020. Nucleic Acids Res. 1997. PMID: 9115371 Free PMC article.
-
Boron neutron capture therapy of cancer: current status and future prospects.Clin Cancer Res. 2005 Jun 1;11(11):3987-4002. doi: 10.1158/1078-0432.CCR-05-0035. Clin Cancer Res. 2005. PMID: 15930333 Review.
-
Boron in cancer therapeutics: An overview.Pharmacol Ther. 2023 Nov;251:108548. doi: 10.1016/j.pharmthera.2023.108548. Epub 2023 Oct 17. Pharmacol Ther. 2023. PMID: 37858628 Review.
Cited by
-
Synthesis and Properties of α-Phosphate-Modified Nucleoside Triphosphates.Molecules. 2024 Aug 30;29(17):4121. doi: 10.3390/molecules29174121. Molecules. 2024. PMID: 39274969 Free PMC article. Review.
-
In vitro evolution of chemically-modified nucleic acid aptamers: Pros and cons, and comprehensive selection strategies.RNA Biol. 2016 Dec;13(12):1232-1245. doi: 10.1080/15476286.2016.1236173. Epub 2016 Oct 7. RNA Biol. 2016. PMID: 27715478 Free PMC article. Review.
-
G-quadruplex induced stabilization by 2'-deoxy-2'-fluoro-D-arabinonucleic acids (2'F-ANA).Nucleic Acids Res. 2007;35(15):4977-88. doi: 10.1093/nar/gkm520. Epub 2007 Jul 17. Nucleic Acids Res. 2007. PMID: 17636049 Free PMC article.
-
Nucleic Acid Aptamers: Emerging Applications in Medical Imaging, Nanotechnology, Neurosciences, and Drug Delivery.Int J Mol Sci. 2017 Nov 16;18(11):2430. doi: 10.3390/ijms18112430. Int J Mol Sci. 2017. PMID: 29144411 Free PMC article. Review.
-
New NTP analogs: the synthesis of 4'-thioUTP and 4'-thioCTP and their utility for SELEX.Nucleic Acids Res. 2005 May 24;33(9):2942-51. doi: 10.1093/nar/gki578. Print 2005. Nucleic Acids Res. 2005. PMID: 15914669 Free PMC article.
References
-
- Barth R., Soloway,A., Goodman,J., Gahbauer,R., Gupta,N., Blue,T., Yang,W. and Tjarks,W. (1999) Boron neutron capture therapy of brain tumors: an emerging therapeutic modality. Neurosurger y, 44, 433–451. - PubMed
-
- Hawthorne M.F. (1998) New horizons for therapy based on the boron neutron capture reaction. Mol. Med. Today, 4, 174–181. - PubMed
-
- Coderre J. and Morris,G. (1999) The radiation biology of boron neutron capture therapy. Radiat. Res., 151, 1–18. - PubMed
-
- Romig T.S., Bell,C. and Drolet,D.W. (1999) Aptamer affinity chromatography: combinatorial chemistry applied to protein purification. J. Chromatogr. B Biomed. Sci. Appl., 731, 275–284. - PubMed
-
- Famulok M. and Mayer,G. (1999) Aptamers as tools in molecular biology and immunology. Curr. Top. Microbiol. Immunol., 243, 123–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

