Cryptochrome light signals control development to suppress auxin sensitivity in the moss Physcomitrella patens
- PMID: 11884681
- PMCID: PMC152919
- DOI: 10.1105/tpc.010388
Cryptochrome light signals control development to suppress auxin sensitivity in the moss Physcomitrella patens
Abstract
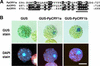
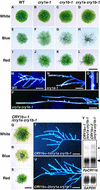
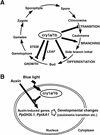
The blue light receptors termed cryptochromes mediate photomorphological responses in seed plants. However, the mechanisms by which cryptochrome signals regulate plant development remain obscure. In this study, cryptochrome functions were analyzed using the moss Physcomitrella patens. This moss has recently become known as the only plant species in which gene replacement occurs at a high frequency by homologous recombination. Two cryptochrome genes were identified in Physcomitrella, and single and double disruptants of these genes were generated. Using these disruptants, it was revealed that cryptochrome signals regulate many steps in moss development, including induction of side branching on protonema and gametophore induction and development. In addition, the disruption of cryptochromes altered auxin responses, including the expression of auxin-inducible genes. Cryptochrome disruptants were more sensitive to external auxin than wild type in a blue light-specific manner, suggesting that cryptochrome light signals repress auxin signals to control plant development.
Figures






References
-
- Abel, S., Nguyen, M.D., and Theologis, A. (1995). The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana. J. Mol. Biol. 251, 533–549. - PubMed
-
- Ahmad, M., and Cashmore, A.R. (1993). HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366, 162–166. - PubMed
-
- Ahmad, M., Lin, C., and Cashmore, A.R. (1995). Mutations throughout an Arabidopsis blue-light photoreceptor impair blue-light-responsive anthocyanin accumulation and inhibition of hypocotyl elongation. Plant J. 8, 653–658. - PubMed
-
- Bhatla, S.C., and Bopp, M. (1985). The hormone regulation of protonema development in mosses. III. Auxin-resistant mutants of the moss Funaria hygrometrica Hedw. J. Plant Physiol. 120, 233–243.
-
- Briggs, W.R., and Huala, E. (1999). Blue-light photoreceptors in higher plants. Annu. Rev. Cell Dev. Biol. 15, 33–62. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

