The dominance of alleles controlling self-incompatibility in Brassica pollen is regulated at the RNA level
- PMID: 11884689
- PMCID: PMC152927
- DOI: 10.1105/tpc.010378
The dominance of alleles controlling self-incompatibility in Brassica pollen is regulated at the RNA level
Abstract
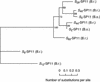
Self-incompatibility (SI) in Brassica is controlled sporophytically by the multiallelic S-locus. The SI phenotype of pollen in an S-heterozygote is determined by the relationship between the two S-haplotypes it carries, and dominant/recessive relationships often are observed between the two S-haplotypes. The S-locus protein 11 (SP11, also known as the S-locus cysteine-rich protein) gene has been cloned from many pollen-dominant S-haplotypes (class I) and shown to encode the pollen S-determinant. However, SP11 from pollen-recessive S-haplotypes (class II) has never been identified by homology-based cloning strategies, and how the dominant/recessive interactions between the two classes occur was not known. We report here the identification and molecular characterization of SP11s from six class II S-haplotypes of B. rapa and B. oleracea. Phylogenetic analysis revealed that the class II SP11s form a distinct group separated from class I SP11s. The promoter sequences and expression patterns of SP11s also were different between the two classes. The mRNA of class II SP11, which was detected predominantly in the anther tapetum in homozygotes, was not detected in the heterozygotes of class I and class II S-haplotypes, suggesting that the dominant/recessive relationships of pollen are regulated at the mRNA level of SP11s.
Figures





Similar articles
-
Linear dominance relationship among four class-II S haplotypes in pollen is determined by the expression of SP11 in Brassica self-incompatibility.Plant Cell Physiol. 2003 Jan;44(1):70-5. doi: 10.1093/pcp/pcg009. Plant Cell Physiol. 2003. PMID: 12552149
-
Trans-acting small RNA determines dominance relationships in Brassica self-incompatibility.Nature. 2010 Aug 19;466(7309):983-6. doi: 10.1038/nature09308. Nature. 2010. PMID: 20725042
-
The pollen determinant of self-incompatibility in Brassica campestris.Proc Natl Acad Sci U S A. 2000 Feb 15;97(4):1920-5. doi: 10.1073/pnas.040556397. Proc Natl Acad Sci U S A. 2000. PMID: 10677556 Free PMC article.
-
Molecular mechanism of self-recognition in Brassica self-incompatibility.J Exp Bot. 2003 Jan;54(380):149-56. doi: 10.1093/jxb/erg007. J Exp Bot. 2003. PMID: 12456765 Review.
-
Pollen recognition and rejection during the sporophytic self-incompatibility response: Brassica and beyond.Trends Plant Sci. 2003 Dec;8(12):606-13. doi: 10.1016/j.tplants.2003.10.007. Trends Plant Sci. 2003. PMID: 14659710 Review.
Cited by
-
Generation of Transgenic Self-Incompatible Arabidopsis thaliana Shows a Genus-Specific Preference for Self-Incompatibility Genes.Plants (Basel). 2019 Dec 4;8(12):570. doi: 10.3390/plants8120570. Plants (Basel). 2019. PMID: 31817214 Free PMC article.
-
Epigenetic mechanisms for breakdown of self-incompatibility in interspecific hybrids.Genetics. 2007 Apr;175(4):1965-73. doi: 10.1534/genetics.106.069393. Epub 2007 Jan 21. Genetics. 2007. PMID: 17237505 Free PMC article.
-
Commonality of self-recognition specificity of S haplotypes between Brassica oleracea and Brassica rapa.Plant Mol Biol. 2003 Jun;52(3):617-26. doi: 10.1023/a:1024819129785. Plant Mol Biol. 2003. PMID: 12956531
-
Evolution of self-incompatibility in the Brassicaceae: Lessons from a textbook example of natural selection.Evol Appl. 2020 Mar 3;13(6):1279-1297. doi: 10.1111/eva.12933. eCollection 2020 Jul. Evol Appl. 2020. PMID: 32684959 Free PMC article.
-
The different mechanisms of sporophytic self-incompatibility.Philos Trans R Soc Lond B Biol Sci. 2003 Jun 29;358(1434):1037-45. doi: 10.1098/rstb.2003.1297. Philos Trans R Soc Lond B Biol Sci. 2003. PMID: 12831470 Free PMC article. Review.
References
-
- Adachi, J., and Hasegawa, M. (1994). MOLPHY: A Computer Program Package for Molecular Phylogenetics, Version 2.3. (Tokyo, Japan: Institute of Statistical Mathematics).
-
- Bateman, A.J. (1955). Self-incompatibility systems in angiosperms. III. Cruciferae. Heredity 9, 52–68.
-
- Brace, J., Ockendon, D.J., and King, G.J. (1993). Development of a method for the identification of S alleles in Brassica oleracea based on digestion of PCR-amplified DNA with restriction endonucleases. Sex. Plant Reprod. 6, 133–138.
-
- Brugière, N., Cui, Y., and Rothstein, S.J. (2000). Molecular mechanisms of self-recognition in Brassica self-incompatibility. Trends Plant Sci. 5, 432–438. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

