Hepatic stellate cells: role in microcirculation and pathophysiology of portal hypertension
- PMID: 11889082
- PMCID: PMC1773176
- DOI: 10.1136/gut.50.4.571
Hepatic stellate cells: role in microcirculation and pathophysiology of portal hypertension
Abstract
Accumulating evidence suggests that stellate cells are involved in the regulation of the liver microcirculation and portal hypertension. Activated hepatic stellate cells have the necessary machinery to contract or relax in response to a number of vasoactive substances. Because stellate cells play a role in both fibrosis and portal hypertension, they are currently regarded as therapeutic targets to prevent and treat the complications of chronic liver disease.
Figures
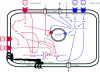
References
-
- Gupta TK, Chen L, Groszmann RJ. Pathophysiology of portal hypertension. Baillieres Clin Gastroenterol 1997;11:203–19. - PubMed
-
- Bhathal PS, Grossmann HJ. Reduction of the increased portal vascular resistance of the isolated perfused rat liver by vasodilators. J Hepatol 1985;1:325–37. - PubMed
-
- Ramadori G. The stellate cell of the liver. Virchows Arch B Cell Pathol 1991;61:147–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
