Metabolic signals that lead to control of CBB gene expression in Rhodobacter capsulatus
- PMID: 11889097
- PMCID: PMC134932
- DOI: 10.1128/JB.184.7.1905-1915.2002
Metabolic signals that lead to control of CBB gene expression in Rhodobacter capsulatus
Abstract
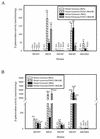
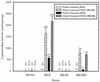
Various mutant strains were used to examine the regulation and metabolic control of the Calvin-Benson-Bassham (CBB) reductive pentose phosphate pathway in Rhodobacter capsulatus. Previously, a ribulose 1,5-bisphosphate carboxylase/oxygenase (RubisCO)-deficient strain (strain SBI/II) was found to show enhanced levels of cbb(I) and cbb(II) promoter activities during photoheterotrophic growth in the presence of dimethyl sulfoxide. With this strain as the starting point, additional mutations were made in genes encoding phosphoribulokinase and transketolase and in the gene encoding the LysR-type transcriptional activator, CbbR(II). These strains revealed that a product generated by phosphoribulokinase was involved in control of CbbR-mediated cbb gene expression in SBI/II. Additionally, heterologous expression experiments indicated that Rhodobacter sphaeroides CbbR responded to the same metabolic signal in R. capsulatus SBI/II and mutant strain backgrounds.
Figures
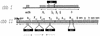

References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1987. Current protocols in molecular biology. Greene Publishing Associates and Wiley Interscience, New York, N.Y.
-
- Barber, R. D., and T. J. Donohue. 1998. Pathways for transcriptional activation of a glutathione-dependent formaldehyde dehydrogenase gene. J. Mol. Biol. 280:775-784. - PubMed
-
- Bauer, C. E., and T. H. Bird. 1996. Regulatory circuits controlling photosynthesis gene expression. Cell 85:5-8. - PubMed
-
- Chen, J. H., J. L. Gibson, L. A. McCue, and F. R. Tabita. 1991. Identification, expression, and deduced primary structure of transketolase and other enzymes encoded within the form II CO2 fixation operon of Rhodobacter sphaeroides. J. Biol. Chem. 30:20447-20452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

