Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p
- PMID: 11889142
- PMCID: PMC2173454
- DOI: 10.1083/jcb.200201002
Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p
Abstract
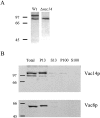
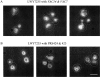
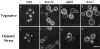
Phosphatidylinositol 3,5-bisphosphate (PtdIns[3,5]P(2)) was first identified as a non-abundant phospholipid whose levels increase in response to osmotic stress. In yeast, Fab1p catalyzes formation of PtdIns(3,5)P(2) via phosphorylation of PtdIns(3)P. We have identified Vac14p, a novel vacuolar protein that regulates PtdIns(3,5)P(2) synthesis by modulating Fab1p activity in both the absence and presence of osmotic stress. We find that PtdIns(3)P levels are also elevated in response to osmotic stress, yet, only the elevation of PtdIns(3,5)P(2) levels are regulated by Vac14p. Under basal conditions the levels of PtdIns(3,5)P(2) are 18-28-fold lower than the levels of PtdIns(3)P, PtdIns(4)P, and PtdIns(4,5)P(2). After a 10 min exposure to hyperosmotic stress the levels of PtdIns(3,5)P(2) rise 20-fold, bringing it to a cellular concentration that is similar to the other phosphoinositides. This suggests that PtdIns(3,5)P(2) plays a major role in osmotic stress, perhaps via regulation of vacuolar volume. In fact, during hyperosmotic stress the vacuole morphology of wild-type cells changes dramatically, to smaller, more highly fragmented vacuoles, whereas mutants unable to synthesize PtdIns(3,5)P(2) continue to maintain a single large vacuole. These findings demonstrate that Vac14p regulates the levels of PtdIns(3,5)P(2) and provide insight into why PtdIns(3,5)P(2) levels rise in response to osmotic stress.
Figures




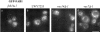

References
-
- Altschul, S.F., W. Gish, W. Miller, E.W. Myers, and D.J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410. - PubMed
-
- Barylko, B., D. Binns, K.M. Lin, M.A. Atkinson, D.M. Jameson, H.L. Yin, and J.P. Albanesi. 1998. Synergistic activation of dynamin GTPase by Grb2 and phosphoinositides. J. Biol. Chem. 273:3791–3797. - PubMed
-
- Beck, K.A., and J.H. Keen. 1991. Interaction of phosphoinositide cycle intermediates with the plasma membrane-associated clathrin assembly protein AP-2. J. Biol. Chem. 266:4442–4447. - PubMed
-
- Berridge, M.J. 1993. Inositol trisphosphate and calcium signalling. Nature. 361:315–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

