Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass
- PMID: 11891119
- PMCID: PMC2819118
- DOI: 10.1016/s0955-0674(02)00310-1
Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass
Abstract
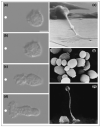
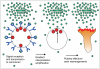
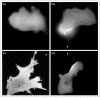
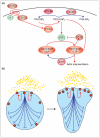
Phosphatidylinositol 3-kinase lipid products and the Rho GTPases play a central role in transmitting information from chemotactic receptors to the effectors of cell polarity, and recent advances in the field have allowed us to understand these roles more clearly. Emergent properties of positive and negative regulation of these molecules may account for the establishment of cell polarity during chemotaxis for a wide range of cells from Dictyostelium to fibroblasts to neutrophils.
Figures
References
-
-
Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–1040.See annotation Haugh et al. (2000) [8••].
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

