Laser capture microdissection and two-dimensional polyacrylamide gel electrophoresis: evaluation of tissue preparation and sample limitations
- PMID: 11891180
- PMCID: PMC1867173
- DOI: 10.1016/S0002-9440(10)64904-8
Laser capture microdissection and two-dimensional polyacrylamide gel electrophoresis: evaluation of tissue preparation and sample limitations
Abstract
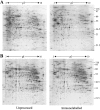
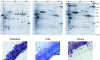
Laser capture microdissection (LCM) is now well established as a tool for facilitating the enrichment of cells of interest from tissue sections, overcoming the problem of tissue heterogeneity. LCM has been used extensively in combination with analysis at the DNA and RNA levels, but only a small number of studies have employed LCM with subsequent protein analysis, albeit with promising results. This study focuses on the potential of LCM in combination with two-dimensional polyacrylamide gel electrophoresis. The effects of tissue section preparation and sample type were evaluated to fully determine the suitability of using LCM in global protein profiling. The effects of several histochemical stains (hematoxylin and eosin, methyl green and toluidine blue) and immunolabeling on subsequent two-dimensional polyacrylamide gel electrophoresis were investigated. Quantitative analysis was performed to establish the extent of changes in the relative intensity of protein species and their reproducibility. All staining protocols tested were found to be compatible with protein analysis although there was variation in protein recovery and the quality of the protein profiles obtained. LCM of renal and cervix samples indicated that protein yield after dissection was acceptable, although the extent of enrichment and dissection time was tissue-dependent, which may preclude the use of this approach with some tissue types. These results indicate that LCM has potential as a tool in proteomic research.
Figures




References
-
- Reymond MA, Sanchez JC, Hughes GJ, Günther K, Riese J, Tortola S, Peinado MA, Kirchner T, Hohenberger W, Hochstrasser DF, Köckerling F: Standardized characterization of gene expression in human colorectal epithelium by two-dimensional electrophoresis. Electrophoresis 1997, 18:2842-2848 - PubMed
-
- Sarto C, Marocchi A, Sanchez JC, Giannone D, Frutiger S, Golaz O, Wilkins MR, Doro G, Cappellano F, Hughes G, Hochstrasser DF, Mocarelli P: Renal cell carcinoma and normal kidney protein expression. Electrophoresis 1997, 18:599-604 - PubMed
-
- Sirivatanauksorn Y, Drury R, Crnogorac-Jurcevic T, Sirivatanauksorn V, Lemoine NR: Laser-assisted microdissection: applications in molecular pathology. J Pathol 1999, 189:150-154 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

