High frequency of t(14;18)-translocation breakpoints outside of major breakpoint and minor cluster regions in follicular lymphomas: improved polymerase chain reaction protocols for their detection
- PMID: 11891181
- PMCID: PMC1867177
- DOI: 10.1016/S0002-9440(10)64905-X
High frequency of t(14;18)-translocation breakpoints outside of major breakpoint and minor cluster regions in follicular lymphomas: improved polymerase chain reaction protocols for their detection
Abstract
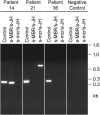
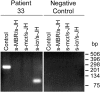
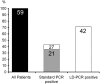
The detection of t(14;18) translocations is widely used for the diagnosis and monitoring of follicular lymphomas displaying a high prevalence for this aberration. Cytogenetics, Southern blotting, and polymerase chain reaction (PCR) are commonly used techniques. It is generally believed that the vast majority of the breakpoints occurs on chromosome 18 in the major breakpoint region (mbr) and the minor cluster region (mcr). Yet, by improving long-distance PCR protocols we identified half of the breakpoints outside of these clusters. Our study included biopsies from 59 patients with follicular lymphoma. Seventy-one percent carried translocations detectable with our long-distance PCR protocol. The novel primer sets were derived from the hitherto uncharacterized 25-kb-long stretch between mbr and mcr that we have sequenced for this purpose. Sequence analysis of the novel breakpoints reveals a wide distribution between mbr and mcr displaying some clustering 16 kb downstream from the BCL2 gene. By including a primer for this intermediate cluster region in standard PCRs we could also improve the detection of t(14;18) translocations in formalin-fixed and paraffin-embedded biopsies. Our new PCRs are highly sensitive, easy to perform, and thus well suited for routine analysis of t(14;18) translocations for the primary diagnosis of follicular lymphoma and surveillance of minimal residual disease.
Figures



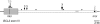
Similar articles
-
Efficacy of screening the intermediate cluster region of the bcl2 gene in follicular lymphomas by PCR.J Clin Pathol. 2005 Jan;58(1):81-2. doi: 10.1136/jcp.2004.018135. J Clin Pathol. 2005. PMID: 15623489 Free PMC article.
-
Sensitivity and reproducibility of conventional qualitative and quantitative PCR assays for detection of the t(14;18)(q32;q21) chromosomal translocation in biopsy material from patients with follicular lymphoma.Int J Mol Med. 2009 Jan;23(1):9-15. Int J Mol Med. 2009. PMID: 19082502
-
Polymerase chain reaction based gene rearrangement studies in the diagnosis of follicular lymphoma--performance in formaldehyde-fixed tissue and application in clinical problem cases.Pathol Res Pract. 1997;193(1):9-19. doi: 10.1016/S0344-0338(97)80089-1. Pathol Res Pract. 1997. PMID: 9112269
-
t(2;18) and t(18;22) variant chromosomal translocations and bcl-2 gene rearrangements in human malignant lymphomas.Nouv Rev Fr Hematol (1978). 1990;32(6):401-3. Nouv Rev Fr Hematol (1978). 1990. PMID: 2129304 Review.
-
[Detection of residual disease in follicular lymphomas using the PCR technique: importance of clono-specific probes].Bull Cancer. 1998 Oct;85(10):847-54. Bull Cancer. 1998. PMID: 9835862 Review. French.
Cited by
-
Presence of t(14;18) positive cells in blood and bone marrow does not predict outcome in follicular lymphoma.Med Oncol. 2009;26(1):16-21. doi: 10.1007/s12032-008-9071-1. Epub 2008 May 10. Med Oncol. 2009. PMID: 18470485
-
High frequency of BCL2 translocation in Thai patients with follicular lymphomas.Int J Hematol. 2007 Nov;86(4):352-7. doi: 10.1532/IJH97.A20709. Int J Hematol. 2007. PMID: 18055344
-
Primary follicular lymphoma of the duodenum.Proc (Bayl Univ Med Cent). 2015 Jul;28(3):381-3. doi: 10.1080/08998280.2015.11929284. Proc (Bayl Univ Med Cent). 2015. PMID: 26130897 Free PMC article.
-
Role of polymerase chain reaction and immunocytochemistry in the cytological assessment of lymphoid proliferations.J Clin Pathol. 2006 Nov;59(11):1160-5. doi: 10.1136/jcp.2005.032987. Epub 2006 Mar 13. J Clin Pathol. 2006. PMID: 16533955 Free PMC article.
-
Mechanism of fragility at BCL2 gene minor breakpoint cluster region during t(14;18) chromosomal translocation.J Biol Chem. 2012 Mar 16;287(12):8688-701. doi: 10.1074/jbc.M111.307363. Epub 2012 Jan 24. J Biol Chem. 2012. PMID: 22275374 Free PMC article.
References
-
- Fukuhara S, Rowley JD, Variakojis D, Golomb HM: Chromosome abnormalities in poorly differentiated lymphocytic lymphoma. Cancer Res 1979, 39:3119-3128 - PubMed
-
- Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM: Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 1984, 226:1097-1099 - PubMed
-
- Korsmeyer SJ: BCL-2 gene family and the regulation of programmed cell death. Cancer Res 1999, 59:1693s-1700s - PubMed
-
- Kroemer G: The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med 1997, 3:614-620 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

