Thymosin-beta4 regulates motility and metastasis of malignant mouse fibrosarcoma cells
- PMID: 11891186
- PMCID: PMC1867176
- DOI: 10.1016/s0002-9440(10)64910-3
Thymosin-beta4 regulates motility and metastasis of malignant mouse fibrosarcoma cells
Abstract
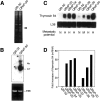
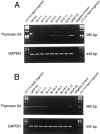
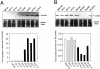
We identified a thymosin-beta4 gene overexpression in malignant mouse fibrosarcoma cells (QRsP-30) that were derived from clonal weakly tumorigenic and nonmetastatic QR-32 cells by using a differential display method. Thymosin-beta4 is known as a 4.9-kd polypeptide that interacts with G-actin and functions as a major actin-sequestering protein in cells. All of the six malignant fibrosarcoma cell lines that have been independently converted from QR-32 cells expressed high levels of thymosin-beta4 mRNA and its expression in tumor cells was correlated with tumorigenicity and metastatic potential. Up-regulation of thymosin-beta4 in QR-32 cells (32-S) transfected with sense thymosin-beta4 cDNA converted the cells to develop tumors and formed numerous lung metastases in syngeneic C57BL/6 mice. In contrast, antisense thymosin-beta4 cDNA-transfected QRsP-30 (30-AS) cells reduced thymosin-beta4 expression, and significantly lost tumor formation and metastases to distant organs. Vector-alone transfected cells (32-V or 30-V cells) behaved like their parental cells. We observed that tumor cell motility, cell shape, and F-actin organization is regulated in proportion to the level of thymosin-beta4 expression. These findings indicate that thymosin-beta4 molecule regulates fibrosarcoma cell tumorigenicity and metastasis through actin-based cytoskeletal organization.
Figures
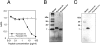
Similar articles
-
Role of thymosin beta4 in tumor metastasis and angiogenesis.J Natl Cancer Inst. 2003 Nov 19;95(22):1674-80. doi: 10.1093/jnci/djg100. J Natl Cancer Inst. 2003. PMID: 14625258
-
Increased E1AF expression in mouse fibrosarcoma promotes metastasis through induction of MT1-MMP expression.Oncogene. 1999 Mar 4;18(9):1771-6. doi: 10.1038/sj.onc.1202465. Oncogene. 1999. PMID: 10208438
-
Thymosin beta4 is a determinant of the transformed phenotype and invasiveness of S-adenosylmethionine decarboxylase-transfected fibroblasts.Cancer Res. 2006 Jan 15;66(2):701-12. doi: 10.1158/0008-5472.CAN-05-2421. Cancer Res. 2006. PMID: 16423999
-
Thymosin β4: a potential molecular target for tumor therapy.Crit Rev Eukaryot Gene Expr. 2012;22(2):109-16. doi: 10.1615/critreveukargeneexpr.v22.i2.30. Crit Rev Eukaryot Gene Expr. 2012. PMID: 22856429 Review.
-
Plasticity of CD44s expression during progression and metastasis of fibrosarcoma in an animal model system.Front Biosci. 1998 Jul 1;3:d672-83. doi: 10.2741/a312. Front Biosci. 1998. PMID: 9634540 Review.
Cited by
-
Effects of maternal separation and methamphetamine exposure on protein expression in the nucleus accumbens shell and core.Metab Brain Dis. 2012 Sep;27(3):363-75. doi: 10.1007/s11011-012-9295-9. Epub 2012 Mar 28. Metab Brain Dis. 2012. PMID: 22451087
-
The role of nicotinamide adenine dinucleotide phosphate oxidase-derived reactive oxygen species in the acquisition of metastatic ability of tumor cells.Am J Pathol. 2006 Jul;169(1):294-302. doi: 10.2353/ajpath.2006.060073. Am J Pathol. 2006. PMID: 16816381 Free PMC article.
-
Verifiable hypotheses for thymosin β4-dependent and -independent angiogenic induction of Trichinella spiralis-triggered nurse cell formation.Int J Mol Sci. 2013 Nov 29;14(12):23492-8. doi: 10.3390/ijms141223492. Int J Mol Sci. 2013. PMID: 24351861 Free PMC article. Review.
-
Thymosin β4 promotes hepatoblastoma metastasis via the induction of epithelial-mesenchymal transition.Mol Med Rep. 2015 Jul;12(1):127-32. doi: 10.3892/mmr.2015.3359. Epub 2015 Feb 16. Mol Med Rep. 2015. PMID: 25695679 Free PMC article.
-
TMSB10 acts as a biomarker and promotes progression of clear cell renal cell carcinoma.Int J Oncol. 2020 May;56(5):1101-1114. doi: 10.3892/ijo.2020.4991. Epub 2020 Feb 19. Int J Oncol. 2020. PMID: 32319572 Free PMC article.
References
-
- Steeg PS, Bevilacqua G, Kopper L, Thorgeirsson UP, Talmadge JE, Liotta LA, Sobel ME: Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst 1988, 80:200-204 - PubMed
-
- Lee J-H, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR: KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst 1996, 88:1731-1737 - PubMed
-
- Ebralidze A, Tulchinsky E, Grigorian M, Afanasyeva A, Senin V, Revazova E, Lukanidin E: Isolation and characterization of a gene specifically expressed in different metastatic cells and whose deduced gene product has a high degree of homology to a Ca2+-binding protein family. Genes Dev 1989, 3:1086-1093 - PubMed
-
- Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P: A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 1991, 65:13-24 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

