Expression of cyclooxygenase 2 is an independent prognostic factor in human ovarian carcinoma
- PMID: 11891188
- PMCID: PMC1867167
- DOI: 10.1016/S0002-9440(10)64912-7
Expression of cyclooxygenase 2 is an independent prognostic factor in human ovarian carcinoma
Abstract
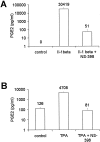
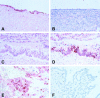
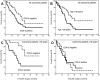
Cyclooxygenase-2 (COX-2) is the rate-limiting enzyme in prostanoid biosynthesis and is involved in tumor progression. We investigated expression of COX-1 and COX-2 in cell lines and tumors from ovarian carcinomas. Expression of COX-2 mRNA and protein was detectable in three of five ovarian carcinoma cell lines and was inducible by interleukin-1beta or phorbolester in a subset of cell lines. Prostaglandin E(2) (PGE(2)) production could be inhibited by the selective COX-2 inhibitor NS-398. In malignant ascites of ovarian carcinomas significantly increased levels of PGE(2) were found compared to other carcinomas or nonmalignant ascites (P = 0.03). We investigated expression of COX-2 by immunohistochemistry in 117 ovarian surface epithelial tumors. Expression of COX-2 was detected in 42% of 86 ovarian carcinomas and in 37% of 19 low malignant potential tumors, but not in 12 cystadenomas or 2 normal ovaries. Expression of COX-1 was detected by immunohistochemistry in 75% of 75 invasive ovarian carcinomas and in 75% of 16 low malignant potential tumors, whereas 2 samples from normal ovaries and 8 cystadenomas were positive for COX-1. In univariate survival analysis of invasive carcinomas, expression of COX-2 was associated with a significantly reduced median survival time (log rank test, P = 0.04). For patients younger than 60 years of age, this association was even more significant (P < 0.004). In contrast, expression of COX-1 was no prognostic parameter (P = 0.89). There was no significant correlation between COX-2 or COX-1 expression and other clinicopathological markers. In multivariate analysis expression of COX-2 was an independent prognostic factor for poor survival (relative risk, 2.74; 95% CI, 1.38 to 5.47). Our data indicate that COX-2 expression is an independent prognostic factor in ovarian carcinoma. Based on the results of this study, it would be interesting to investigate whether ovarian carcinoma patients with tumors positive for COX-2 would benefit from treatment with selective COX-2 inhibitors.
Figures




Comment in
-
The function of COX-2 in human ovarian carcinoma.Am J Pathol. 2003 Jul;163(1):368; author reply 368-9. doi: 10.1016/s0002-9440(10)63661-9. Am J Pathol. 2003. PMID: 12819042 Free PMC article. No abstract available.
Similar articles
-
[Relationship of cyclooxygenase 2 expression and chemotherapy response and prognosis in human ovarian carcinoma].Zhonghua Fu Chan Ke Za Zhi. 2004 Aug;39(8):529-32. Zhonghua Fu Chan Ke Za Zhi. 2004. PMID: 15363351 Chinese.
-
Expression of cyclooxygenase-2 in association with clinicopathological prognostic factors and molecular markers in epithelial ovarian cancer.Gynecol Oncol. 2004 Mar;92(3):927-35. doi: 10.1016/j.ygyno.2003.11.055. Gynecol Oncol. 2004. PMID: 14984962
-
Overexpression of the embryonic-lethal abnormal vision-like protein HuR in ovarian carcinoma is a prognostic factor and is associated with increased cyclooxygenase 2 expression.Cancer Res. 2004 Jan 1;64(1):189-95. doi: 10.1158/0008-5472.can-03-1987. Cancer Res. 2004. PMID: 14729623
-
The effect of cyclooxygenase-2 expression on tumor vascularity in advanced stage ovarian serous carcinoma.Cancer. 2003 Oct 1;98(7):1423-9. doi: 10.1002/cncr.11650. Cancer. 2003. PMID: 14508829
-
Prognostic role of cyclooxygenase-2 in epithelial ovarian cancer: a meta-analysis of observational studies.Gynecol Oncol. 2013 Jun;129(3):613-9. doi: 10.1016/j.ygyno.2013.02.011. Epub 2013 Feb 17. Gynecol Oncol. 2013. PMID: 23422504 Review.
Cited by
-
Molecular and Cellular Mechanisms of Antitumor Immune Response Activation by Dendritic Cells.Acta Naturae. 2016 Jul-Sep;8(3):17-30. Acta Naturae. 2016. PMID: 27795841 Free PMC article.
-
Diverse mechanisms for activation of Wnt signalling in the ovarian tumour microenvironment.Biochem J. 2011 Jul 1;437(1):1-12. doi: 10.1042/BJ20110112. Biochem J. 2011. PMID: 21668411 Free PMC article. Review.
-
Analysis of cyclooxygenase-2 expression in human breast cancer: high throughput tissue microarray analysis.J Cancer Res Clin Oncol. 2003 Jul;129(7):375-82. doi: 10.1007/s00432-003-0459-1. Epub 2003 Jul 15. J Cancer Res Clin Oncol. 2003. PMID: 12884024 Free PMC article.
-
Age dependent increase in prostaglandin pathway coincides with onset of ovarian cancer in laying hens.Prostaglandins Leukot Essent Fatty Acids. 2012 Dec;87(6):177-84. doi: 10.1016/j.plefa.2012.09.003. Epub 2012 Oct 23. Prostaglandins Leukot Essent Fatty Acids. 2012. PMID: 23089186 Free PMC article.
-
High and low frequency subharmonic imaging of angiogenesis in a murine breast cancer model.Ultrasonics. 2015 Sep;62:50-5. doi: 10.1016/j.ultras.2015.04.012. Epub 2015 May 5. Ultrasonics. 2015. PMID: 25979676 Free PMC article.
References
-
- Greenlee RT, Murray T, Bolden S, Wingo PA: Cancer statistics. Ca Cancer J Clin 2000, 50:7-33 - PubMed
-
- Thun MJ, Namboodiri MM, Heath Jr CW: Aspirin use and reduced risk of fatal colon cancer. N Engl J Med 1991, 325:1593–1596 - PubMed
-
- Schreinemachers DM, Everson RB: Aspirin use and lung, colon and breast cancer incidence in a prospective study. Epidemiology 1994, 5:138-146 - PubMed
-
- Taketo MM: Cyclooxygenase inhibitors in tumorigenesis (part I). J Natl Cancer Inst 1998, 90:1529-1536 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

