Extensive induction of important mediators of fibrosis and dystrophic calcification in desmin-deficient cardiomyopathy
- PMID: 11891192
- PMCID: PMC1867168
- DOI: 10.1016/S0002-9440(10)64916-4
Extensive induction of important mediators of fibrosis and dystrophic calcification in desmin-deficient cardiomyopathy
Abstract
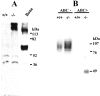
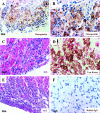
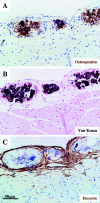
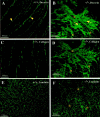
Mice lacking the intermediate filament protein desmin demonstrate abnormal mitochondria behavior, disruption of muscle architecture, and myocardial degeneration with extensive calcium deposits and fibrosis. These abnormalities are associated with cardiomyocyte hypertrophy, cardiac chamber dilation and eventually with heart failure. In an effort to elucidate the molecular mechanisms leading to the observed pathogenesis, we have analyzed gene expression changes in cardiac tissue using differential display polymerase chain reaction and cDNA atlas array methods. The most substantial changes were found in genes coding the small extracellular matrix proteins osteopontin and decorin that are dramatically induced in the desmin-null myocardium. We further analyzed their expression pattern both at the RNA and protein levels and we compared their spatial expression with the onset of calcification. Extensive osteopontin localization is observed by immunohistochemistry in the desmin-null myocardium in areas with massive myocyte death, as well as in hypercellular regions with variable degrees of calcification and fibrosis. Osteopontin is consistently co-localized with calcified deposits, which progressively are transformed to psammoma bodies surrounded by decorin, especially in the right ventricle. These data together with the observed up-regulation of transforming growth factor-beta1 and angiotensin-converting enzyme, could explain the extensive fibrosis and dystrophic calcification observed in the heart of desmin-null mice, potentially crucial events leading to heart failure.
Figures



Similar articles
-
Combined deficiency of dystrophin and beta1 integrin in the cardiac myocyte causes myocardial dysfunction, fibrosis and calcification.Circ Res. 2008 May 9;102(9):1109-17. doi: 10.1161/CIRCRESAHA.108.173153. Epub 2008 Mar 13. Circ Res. 2008. PMID: 18340010
-
Cardiomyocyte-specific desmin rescue of desmin null cardiomyopathy excludes vascular involvement.J Mol Cell Cardiol. 2004 Jan;36(1):121-8. doi: 10.1016/j.yjmcc.2003.10.010. J Mol Cell Cardiol. 2004. PMID: 14734054
-
Bcl-2 overexpression corrects mitochondrial defects and ameliorates inherited desmin null cardiomyopathy.Proc Natl Acad Sci U S A. 2004 Jan 20;101(3):769-74. doi: 10.1073/pnas.0303202101. Epub 2004 Jan 8. Proc Natl Acad Sci U S A. 2004. PMID: 14715896 Free PMC article.
-
Pathophysiological mechanisms of cardiomyopathies induced by desmin gene variants located in the C-Terminus of segment 2B.J Cell Physiol. 2024 May;239(5):e31254. doi: 10.1002/jcp.31254. Epub 2024 Mar 19. J Cell Physiol. 2024. PMID: 38501553 Review.
-
Desmin-related myopathies in mice and man.Acta Physiol Scand. 2001 Mar;171(3):341-8. doi: 10.1046/j.1365-201x.2001.00837.x. Acta Physiol Scand. 2001. PMID: 11412147 Review.
Cited by
-
Common and Key Differential Pathogenic Pathways in Desminopathy and Titinopathy.Int J Med Sci. 2024 Aug 1;21(11):2040-2051. doi: 10.7150/ijms.97797. eCollection 2024. Int J Med Sci. 2024. PMID: 39239540 Free PMC article.
-
Μyospryn: a multifunctional desmin-associated protein.Histochem Cell Biol. 2013 Jul;140(1):55-63. doi: 10.1007/s00418-013-1103-z. Epub 2013 Jun 9. Histochem Cell Biol. 2013. PMID: 23748244 Review.
-
Desmin and αB-crystallin interplay in the maintenance of mitochondrial homeostasis and cardiomyocyte survival.J Cell Sci. 2016 Oct 15;129(20):3705-3720. doi: 10.1242/jcs.192203. Epub 2016 Aug 26. J Cell Sci. 2016. PMID: 27566162 Free PMC article.
-
Myospryn deficiency leads to impaired cardiac structure and function and schizophrenia-associated symptoms.Cell Tissue Res. 2021 Sep;385(3):675-696. doi: 10.1007/s00441-021-03447-2. Epub 2021 May 26. Cell Tissue Res. 2021. PMID: 34037836
-
Desmin Reorganization by Stimuli Inducing Oxidative Stress and Electrophiles: Role of Its Single Cysteine Residue.Antioxidants (Basel). 2023 Aug 31;12(9):1703. doi: 10.3390/antiox12091703. Antioxidants (Basel). 2023. PMID: 37760006 Free PMC article.
References
-
- Milner DJ, Taffet GE, Wang X, Pham T, Tamura T, Hartley C, Gerdes AM, Capetanaki Y: The absence of desmin leads to cardiomyocyte hypertrophy and cardiac dilation with compromised systolic function. J Mol Cell Cardiol 1999, 31:2063-2076 - PubMed
-
- Li Z, Colucci-Guyon E, Pincon-Raymond M, Mericskay M, Pournin S, Paulin D, Babinet C: Cardiovascular lesions and skeletal myopathy in mice lacking desmin. Dev Biol 1996, 175:362-366 - PubMed
-
- Li D, Tapscoft T, Gonzalez O, Burch PE, Quinones MA, Zoghbi WA, Hill R, Bachinski LL, Mann DL, Roberts R: Desmin mutation responsible for idiopathic dilated cardiomyopathy. Circulation 1999, 100:461-464 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

