Fibrinogen stabilizes placental-maternal attachment during embryonic development in the mouse
- PMID: 11891199
- PMCID: PMC1867160
- DOI: 10.1016/S0002-9440(10)64923-1
Fibrinogen stabilizes placental-maternal attachment during embryonic development in the mouse
Abstract
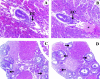
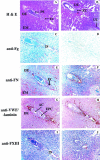
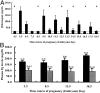
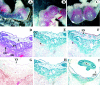
In humans, maternal fibrinogen (Fg) is required to support pregnancies by maintaining hemostatic balance and stabilizing uteroplacental attachment at the fibrinoid layer found at the fetal-maternal junction. To examine relationships between low Fg levels and early fetal loss, a genetic model of afibrinogenemia was developed. Pregnant mice homozygous for a deletion of the Fg-gamma chain, which results in a total Fg deficiency state (FG(-/-)), aborted the fetuses at the equivalent gestational stage seen in humans. Results obtained from timed matings of FG(-/-) mice showed that vaginal bleeding was initiated as early as embryonic day (E)6 to 7, a critical stage for maternal-fetal vascular development. The condition of afibrinogenemia retarded embryo-placental development, and consistently led to abortion and maternal death at E9.75. Lack of Fg did not alter the extent or distribution pattern of other putative factors of embryo-placental attachment, including laminin, fibronectin, and Factor XIII, indicating that the presence of fibrin(ogen) is required to confer sufficient stability at the placental-decidual interface. The results of these studies demonstrate that maternal Fg plays a critical role in maintenance of pregnancy in mice, both by supporting proper development of fetal-maternal vascular communication and stabilization of embryo implantation.
Figures




Similar articles
-
Studies on the role of adhesive proteins in maintaining pregnancy.Horm Res. 1998;50 Suppl 2:37-45. doi: 10.1159/000053122. Horm Res. 1998. PMID: 9721590
-
Coagulation factor deficiencies and pregnancy loss.Semin Thromb Hemost. 2003 Apr;29(2):171-4. doi: 10.1055/s-2003-38832. Semin Thromb Hemost. 2003. PMID: 12709920 Review.
-
Maternal fibrinogen is necessary for embryonic development.Curr Drug Targets. 2005 Aug;6(5):535-9. doi: 10.2174/1389450054546006. Curr Drug Targets. 2005. PMID: 16026273 Review.
-
A total fibrinogen deficiency is compatible with the development of pulmonary fibrosis in mice.Am J Pathol. 2000 Sep;157(3):703-8. doi: 10.1016/S0002-9440(10)64582-8. Am J Pathol. 2000. PMID: 10980108 Free PMC article.
-
MicroRNA-30d deficiency during preconception affects endometrial receptivity by decreasing implantation rates and impairing fetal growth.Am J Obstet Gynecol. 2019 Jul;221(1):46.e1-46.e16. doi: 10.1016/j.ajog.2019.02.047. Epub 2019 Feb 28. Am J Obstet Gynecol. 2019. PMID: 30826341
Cited by
-
miRNAs and Their Gene Targets-A Clue to Differentiate Pregnancies with Small for Gestational Age Newborns, Intrauterine Growth Restriction, and Preeclampsia.Diagnostics (Basel). 2021 Apr 20;11(4):729. doi: 10.3390/diagnostics11040729. Diagnostics (Basel). 2021. PMID: 33923995 Free PMC article.
-
[Pedigree Analysis and Diagnosis of Congenital Dysfibrinogenemia: A Case Report].Sichuan Da Xue Xue Bao Yi Xue Ban. 2022 Jan;53(1):171-174. doi: 10.12182/20220160201. Sichuan Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 35048620 Free PMC article. Chinese.
-
The Fgl2/fibroleukin prothrombinase contributes to immunologically mediated thrombosis in experimental and human viral hepatitis.J Clin Invest. 2003 Jul;112(1):58-66. doi: 10.1172/JCI18114. J Clin Invest. 2003. PMID: 12840059 Free PMC article.
-
Management of hemophagocytic lymphohistiocytosis in pregnancy: Case series study and literature review.J Obstet Gynaecol Res. 2022 Mar;48(3):610-620. doi: 10.1111/jog.15133. Epub 2022 Jan 2. J Obstet Gynaecol Res. 2022. PMID: 34978123 Free PMC article. Review.
-
Natural history of patients with congenital dysfibrinogenemia.Blood. 2015 Jan 15;125(3):553-61. doi: 10.1182/blood-2014-06-582866. Epub 2014 Oct 15. Blood. 2015. PMID: 25320241 Free PMC article.
References
-
- Doolittle RF: Fibrinogen and fibrin. Annu Rev Biochem 1984, 53:195-229 - PubMed
-
- McRitchie DI, Girotti MJ, Glynn MF, Goldberg JM, Rotstein OD: Effect of systemic fibrinogen depletion on intraabdominal abscess formation. J Lab Clin Med 1991, 118:48-55 - PubMed
-
- Kuijper PH, Gallardo-Torres HI, Lammers JW, Sixma JJ, Koenderman L, Zwaginga JJ: Platelet and fibrin deposition at the damaged vessel wall: cooperative substrates for neutrophil adhesion under flow conditions. Blood 1997, 89:166-175 - PubMed
-
- Martin P: Wound healing—aiming for perfect skin regeneration. Science 1997, 276:75-81 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

