Latent sensitivity to Fas-mediated apoptosis after CD40 ligation may explain activity of CD154 gene therapy in chronic lymphocytic leukemia
- PMID: 11891278
- PMCID: PMC122613
- DOI: 10.1073/pnas.022604399
Latent sensitivity to Fas-mediated apoptosis after CD40 ligation may explain activity of CD154 gene therapy in chronic lymphocytic leukemia
Abstract
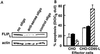
Patients with chronic lymphocytic leukemia (CLL) treated with adenovirus (Ad)-CD154 (CD40L) gene therapy experience reductions in leukemia cell counts and lymph node size associated with induction of the death receptor Fas (CD95). CD4 T cell lines can induce apoptosis of CD40-activated CLL cells via a CD95 ligand (CD95-L)-dependent mechanism. To examine whether CD95-L was sufficient to induce cytolysis of CD40-activated CLL cells, we used Chinese hamster ovary cells transfected with CD95-L as cytotoxic effector cells. CD40-activated CLL cells were initially resistant to CD95-mediated apoptosis despite high-level expression of CD95. However, after 72 h, CLL cells from seven of seven patients became increasingly sensitive to CD95-mediated apoptosis. This sensitivity correlated with a progressive decline in Flice-inhibitory protein (FLIP), which was induced within 24 h of CD40 ligation. Down-regulation of FLIP with an antisense oligonucleotide or a pharmacologic agent, however, was not sufficient to render CLL cells sensitive to CD95-mediated apoptosis in the 24-72 h after CD40 activation. Although the levels of pro-Caspase-8 appeared sufficient, inadequate levels of Fas-associated death domain protein (FADD) and DAP3 may preclude assembly of the death-inducing signaling complex. Seventy-two hours after CD40 ligation, sensitivity to CD95 and a progressive increase in FADD and DAP3 were associated with the acquired ability of FADD and FLIP to coimmunoprecipitate with the death-inducing signaling complex after CD95 ligation. Collectively, these studies reveal that CD40 ligation on CLL B cells induces a programmed series of events in which the cells initially are protected and then sensitized to CD95-mediated apoptosis through shifts in the balance of the anti- and proapoptotic proteins FLIP and FADD.
Figures
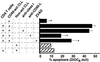

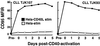




Similar articles
-
Resistance to CD95-mediated apoptosis of CD40-activated chronic lymphocytic leukemia B cells is not related to lack of DISC molecules expression.Hematol J. 2004;5(2):152-60. doi: 10.1038/sj.thj.6200362. Hematol J. 2004. PMID: 15048066
-
Fas-ligand (CD178) and TRAIL synergistically induce apoptosis of CD40-activated chronic lymphocytic leukemia B cells.Blood. 2005 Apr 15;105(8):3193-8. doi: 10.1182/blood-2003-10-3684. Epub 2004 Aug 31. Blood. 2005. PMID: 15339846
-
Metabolic inhibitors sensitize for CD95 (APO-1/Fas)-induced apoptosis by down-regulating Fas-associated death domain-like interleukin 1-converting enzyme inhibitory protein expression.Cancer Res. 2000 Jul 15;60(14):3947-56. Cancer Res. 2000. PMID: 10919673
-
CD154 gene therapy for human B-cell malignancies.Ann N Y Acad Sci. 2005 Dec;1062:51-60. doi: 10.1196/annals.1358.008. Ann N Y Acad Sci. 2005. PMID: 16461788 Review.
-
Inducible resistance to Fas-mediated apoptosis in B cells.Cell Res. 2000 Dec;10(4):245-66. doi: 10.1038/sj.cr.7290053. Cell Res. 2000. PMID: 11191348 Review.
Cited by
-
Characteristics and clinical trial results of agonistic anti-CD40 antibodies in the treatment of malignancies.Oncol Lett. 2020 Nov;20(5):176. doi: 10.3892/ol.2020.12037. Epub 2020 Aug 31. Oncol Lett. 2020. PMID: 32934743 Free PMC article. Review.
-
A phase I study of immune gene therapy for patients with CLL using a membrane-stable, humanized CD154.Leukemia. 2010 Nov;24(11):1893-900. doi: 10.1038/leu.2010.191. Epub 2010 Sep 30. Leukemia. 2010. PMID: 20882050 Free PMC article. Clinical Trial.
-
Primary and malignant cholangiocytes undergo CD40 mediated Fas dependent apoptosis, but are insensitive to direct activation with exogenous Fas ligand.PLoS One. 2010 Nov 17;5(11):e14037. doi: 10.1371/journal.pone.0014037. PLoS One. 2010. PMID: 21103345 Free PMC article.
-
Molecular and cellular mechanisms of CLL: novel therapeutic approaches.Nat Rev Clin Oncol. 2009 Jul;6(7):405-18. doi: 10.1038/nrclinonc.2009.72. Epub 2009 Jun 2. Nat Rev Clin Oncol. 2009. PMID: 19488076 Review.
-
Inhibitors of XIAP sensitize CD40-activated chronic lymphocytic leukemia cells to CD95-mediated apoptosis.Blood. 2005 Sep 1;106(5):1742-8. doi: 10.1182/blood-2005-02-0695. Epub 2005 May 24. Blood. 2005. PMID: 15914559 Free PMC article.
References
-
- Kipps T J. In: Williams Hematology. Beutler E, Lichtman M A, Coller B S, Kipps T J, Seligsohn U, editors. New York: McGraw–Hill; 2001. pp. 1163–1194.
-
- Wierda W G, Cantwell M J, Woods S J, Rassenti L Z, Prussak C E, Kipps T J. Blood. 2000;96:2917–2924. - PubMed
-
- Nagata S. Cell. 1997;88:355–365. - PubMed
-
- Krammer P H. Nature (London) 2000;407:789–795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

