Covalent modification of proteins by cocaine
- PMID: 11891282
- PMCID: PMC122537
- DOI: 10.1073/pnas.042700599
Covalent modification of proteins by cocaine
Abstract
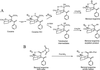
Cocaine covalently modifies proteins through a reaction in which the methyl ester of cocaine acylates the epsilon-amino group of lysine residues. This reaction is highly specific in vitro, because no other amino acid reacts with cocaine, and only cocaine's methyl ester reacts with the lysine side chain. Covalently modified proteins were present in the plasma of rats and human subjects chronically exposed to cocaine. Modified endogenous proteins are immunogenic, and specific antibodies were elicited in mouse and detected in the plasma of human subjects. Covalent modification of proteins could explain cocaine's autoimmune effects and provide a new biochemical approach to cocaine's long-term actions.
Figures


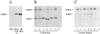

References
-
- Chow M J, Ambre J J, Ruo T I, Atkinson A J, Bowsher D J, Fisherman M W. Clin Pharmacol Ther. 1985;38:318–324. - PubMed
-
- Isenschmid D S, Fischman M W, Foltin R W, Caplan Y H. J Anal Toxicol. 1992;16:311–314. - PubMed
-
- Volkow N D, Hitzemann R, Wang G J, Fowler J S, Wolf A P, Dewey S L, Handlesman L. Synapse. 1992;11:184–190. - PubMed
-
- Volkow N D, Wang G J, Fowler J S, Logan J, Gatley S J, Hitzemann R, Chen A D, Dewey S L, Pappas N. Nature (London) 1997;386:830–833. - PubMed
-
- Karoum F, Suddath R I, Wyatt R J. Eur J Pharmacol. 1990;186:1–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

