Structural studies of the scrapie prion protein by electron crystallography
- PMID: 11891310
- PMCID: PMC122563
- DOI: 10.1073/pnas.052703499
Structural studies of the scrapie prion protein by electron crystallography
Abstract
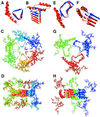
Because the insolubility of the scrapie prion protein (PrP(Sc)) has frustrated structural studies by x-ray crystallography or NMR spectroscopy, we used electron crystallography to characterize the structure of two infectious variants of the prion protein. Isomorphous two-dimensional crystals of the N-terminally truncated PrP(Sc) (PrP 27-30) and a miniprion (PrP(Sc)106) were identified by negative stain electron microscopy. Image processing allowed the extraction of limited structural information to 7 A resolution. By comparing projection maps of PrP 27-30 and PrP(Sc)106, we visualized the 36-residue internal deletion of the miniprion and localized the N-linked sugars. The dimensions of the monomer and the locations of the deleted segment and sugars were used as constraints in the construction of models for PrP(Sc). Only models featuring parallel beta-helices as the key element could satisfy the constraints. These low-resolution projection maps and models have implications for understanding prion propagation and the pathogenesis of neurodegeneration.
Figures



Similar articles
-
Electron crystallography of the scrapie prion protein complexed with heavy metals.Arch Biochem Biophys. 2007 Nov 15;467(2):239-48. doi: 10.1016/j.abb.2007.08.010. Epub 2007 Aug 23. Arch Biochem Biophys. 2007. PMID: 17935686 Free PMC article.
-
Ultrastructural studies on scrapie prion protein crystals obtained from reverse micellar solutions.Biophys J. 1999 Feb;76(2):1048-62. doi: 10.1016/S0006-3495(99)77270-X. Biophys J. 1999. PMID: 9916037 Free PMC article.
-
Biochemistry and structure of PrP(C) and PrP(Sc).Br Med Bull. 2003;66:21-33. doi: 10.1093/bmb/66.1.21. Br Med Bull. 2003. PMID: 14522846 Review.
-
X-ray diffraction of scrapie prion rods and PrP peptides.J Mol Biol. 1995 Sep 29;252(4):412-22. doi: 10.1006/jmbi.1995.0507. J Mol Biol. 1995. PMID: 7563061
-
Transition of the prion protein from a structured cellular form (PrPC ) to the infectious scrapie agent (PrPSc ).Protein Sci. 2019 Dec;28(12):2055-2063. doi: 10.1002/pro.3735. Epub 2019 Oct 25. Protein Sci. 2019. PMID: 31583788 Free PMC article. Review.
Cited by
-
Structural organization of mammalian prions as probed by limited proteolysis.PLoS One. 2012;7(11):e50111. doi: 10.1371/journal.pone.0050111. Epub 2012 Nov 20. PLoS One. 2012. PMID: 23185550 Free PMC article.
-
A structural model for Alzheimer's beta -amyloid fibrils based on experimental constraints from solid state NMR.Proc Natl Acad Sci U S A. 2002 Dec 24;99(26):16742-7. doi: 10.1073/pnas.262663499. Epub 2002 Dec 12. Proc Natl Acad Sci U S A. 2002. PMID: 12481027 Free PMC article.
-
Searching for factors that distinguish disease-prone and disease-resistant prions via sequence analysis.Bioinform Biol Insights. 2008 Mar 12;2:133-44. doi: 10.4137/bbi.s550. Bioinform Biol Insights. 2008. PMID: 19812771 Free PMC article.
-
High-resolution molecular structure of a peptide in an amyloid fibril determined by magic angle spinning NMR spectroscopy.Proc Natl Acad Sci U S A. 2004 Jan 20;101(3):711-6. doi: 10.1073/pnas.0304849101. Epub 2004 Jan 8. Proc Natl Acad Sci U S A. 2004. PMID: 14715898 Free PMC article.
-
Structure-Based Drug Discovery for Prion Disease Using a Novel Binding Simulation.EBioMedicine. 2016 Jul;9:238-249. doi: 10.1016/j.ebiom.2016.06.010. Epub 2016 Jun 8. EBioMedicine. 2016. PMID: 27333028 Free PMC article.
References
-
- Cohen F E, Prusiner S B. In: Prion Biology and Diseases. Prusiner S B, editor. Plainview, NY: Cold Spring Harbor Lab. Press; 1999. pp. 191–228.
-
- Prusiner S B, McKinley M P, Bowman K A, Bolton D C, Bendheim P E, Groth D F, Glenner G G. Cell. 1983;35:349–358. - PubMed
-
- Nguyen J T, Inouye H, Baldwin M A, Fletterick R J, Cohen F E, Prusiner S B, Kirschner D A. J Mol Biol. 1995;252:412–422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

