Cells exposed to antifolates show increased cellular levels of proteins fused to dihydrofolate reductase: a method to modulate gene expression
- PMID: 11891321
- PMCID: PMC122535
- DOI: 10.1073/pnas.062036899
Cells exposed to antifolates show increased cellular levels of proteins fused to dihydrofolate reductase: a method to modulate gene expression
Abstract
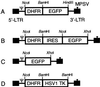
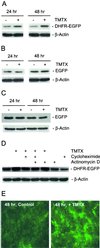
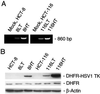
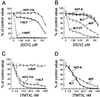
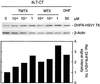
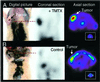
Human cells exposed to antifolates show a rapid increase in the levels of the enzyme dihydrofolate reductase (DHFR). We hypothesized that this adaptive response mechanism can be used to elevate cellular levels of proteins fused to DHFR. In this study, mouse cells transfected to express a green fluorescent protein-DHFR fusion protein and subsequently exposed to the antifolate trimetrexate (TMTX) showed a specific and time-dependent increase in cellular levels of the fusion protein. Next, human HCT-8 and HCT-116 colon cancer cells retrovirally transduced to express a DHFR-herpes simplex virus 1 thymidine kinase (HSV1 TK) fusion protein and treated with the DHFR inhibitor TMTX exhibited increased levels of the DHFR-HSV1 TK fusion protein and an increase in ganciclovir sensitivity by 250-fold. The level of fusion protein in antifolate-treated human tumor cells was increased in response to a 24-h exposure of methotrexate, trimetrexate, as well as dihydrofolate. This effect depended on the antifolate concentration and was independent of the fusion-protein mRNA levels, consistent with this increase occurring at a translational level. In a xenograft model, nude rats bearing DHFR-HSV1 TK-transduced HCT-8 tumors and treated with TMTX showed, after 24 h, a 2- to 4-fold increase of fusion-protein levels in tumor tissue from treated animals compared with controls, as determined by Western blotting. The fusion-protein increase was imaged with positron-emission tomography, where a substantially enhanced signal of the transduced tumor was detected in animals after antifolate administration. Drug-mediated elevation of cellular DHFR-fused proteins is a very useful method to modulate gene expression in vivo for imaging as well as therapeutic purposes.
Figures


Similar articles
-
Imaging of dihydrofolate reductase fusion gene expression in xenografts of human liver metastases of colorectal cancer in living rats.Eur J Nucl Med Mol Imaging. 2003 Sep;30(9):1281-91. doi: 10.1007/s00259-003-1143-z. Epub 2003 Mar 27. Eur J Nucl Med Mol Imaging. 2003. PMID: 12664136
-
Protection of CCRF-CEM human lymphoid cells from antifolates by retroviral gene transfer of variants of murine dihydrofolate reductase.Cancer Gene Ther. 1998 Jul-Aug;5(4):225-35. Cancer Gene Ther. 1998. PMID: 9694074
-
Increased resistance to nitrogen mustards and antifolates following in vitro selection of murine fibroblasts and primary hematopoietic cells transduced with a bicistronic retroviral vector expressing the rat glutathione S-transferase A3 and a mutant dihydrofolate reductase.Cancer Gene Ther. 2003 Aug;10(8):637-46. doi: 10.1038/sj.cgt.7700619. Cancer Gene Ther. 2003. PMID: 12872145
-
Novel aspects of resistance to drugs targeted to dihydrofolate reductase and thymidylate synthase.Biochim Biophys Acta. 2002 Jul 18;1587(2-3):164-73. doi: 10.1016/s0925-4439(02)00079-0. Biochim Biophys Acta. 2002. PMID: 12084458 Review.
-
Molecular mechanisms of resistance to antifolates, a review.Acta Biochim Pol. 1995;42(4):457-64. Acta Biochim Pol. 1995. PMID: 8852336 Review.
Cited by
-
Dihydrofolate Reductase and Thymidylate Synthase Transgenes Resistant to Methotrexate Interact to Permit Novel Transgene Regulation.J Biol Chem. 2015 Sep 18;290(38):22970-6. doi: 10.1074/jbc.C115.671123. Epub 2015 Aug 4. J Biol Chem. 2015. PMID: 26242737 Free PMC article.
-
Molecular-genetic imaging: current and future perspectives.J Clin Invest. 2003 Jun;111(11):1620-9. doi: 10.1172/JCI18855. J Clin Invest. 2003. PMID: 12782662 Free PMC article. Review. No abstract available.
-
Imaging of dihydrofolate reductase fusion gene expression in xenografts of human liver metastases of colorectal cancer in living rats.Eur J Nucl Med Mol Imaging. 2003 Sep;30(9):1281-91. doi: 10.1007/s00259-003-1143-z. Epub 2003 Mar 27. Eur J Nucl Med Mol Imaging. 2003. PMID: 12664136
-
Molecular Imaging with Reporter Genes: Has Its Promise Been Delivered?J Nucl Med. 2019 Dec;60(12):1665-1681. doi: 10.2967/jnumed.118.220004. J Nucl Med. 2019. PMID: 31792128 Free PMC article. Review.
-
Applications of nuclear-based imaging in gene and cell therapy: probe considerations.Mol Ther Oncolytics. 2021 Feb 4;20:447-458. doi: 10.1016/j.omto.2021.01.017. eCollection 2021 Mar 26. Mol Ther Oncolytics. 2021. PMID: 33718593 Free PMC article.
References
-
- Bertino J R, Donohue D R, Garbio B W, Silber R, Alenty A, Mayer M, Huennekens F M. Nature (London) 1962;193:140–141. - PubMed
-
- Bertino J R, Cashmore A R, Hillcoat B L. Cancer Res. 1970;30:2372–2378. - PubMed
-
- Domin B A, Grill S P, Bastow K F, Cheng Y C. Mol Pharmacol. 1982;21:478–482. - PubMed
-
- Ercikan-Abali E A, Banerjee D, Waltham M C, Skacel N, Scotto K W, Bertino J R. Biochemistry. 1997;7:12317–12322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

