Small-molecule antagonists of Myc/Max dimerization inhibit Myc-induced transformation of chicken embryo fibroblasts
- PMID: 11891322
- PMCID: PMC122609
- DOI: 10.1073/pnas.062036999
Small-molecule antagonists of Myc/Max dimerization inhibit Myc-induced transformation of chicken embryo fibroblasts
Abstract
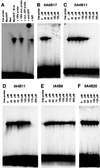
Myc is a transcriptional regulator of the basic helix-loop-helix leucine zipper protein family. It has strong oncogenic potential, mutated or virally transduced forms of Myc induce lymphoid tumors in animals, and deregulated expression of Myc is associated with numerous types of human cancers. For its oncogenic activity, Myc must dimerize with the ubiquitously expressed basic helix-loop-helix leucine zipper protein Max. This requirement for dimerization may allow control of Myc activity with small molecules that interfere with Myc/Max dimerization. We have measured Myc/Max dimerization with fluorescence resonance energy transfer and have screened combinatorial chemical libraries for inhibitors of dimerization. Candidate inhibitors were isolated from a peptidomimetics library. Inhibition of Myc/Max interaction was validated by ELISA and electrophoretic mobility-shift assay. Two of the candidate inhibitors also interfere with Myc-induced oncogenic transformation in chicken embryo fibroblast cultures. Our work provides proof of principle for the identification of small molecule inhibitors of protein-protein interactions by using high-throughput screens of combinatorial chemical libraries.
Figures
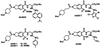


Similar articles
-
Low molecular weight inhibitors of Myc-Max interaction and function.Oncogene. 2003 Sep 18;22(40):6151-9. doi: 10.1038/sj.onc.1206641. Oncogene. 2003. PMID: 13679853
-
Structure, function, and dynamics of the dimerization and DNA-binding domain of oncogenic transcription factor v-Myc.J Mol Biol. 2001 Apr 13;307(5):1395-410. doi: 10.1006/jmbi.2001.4537. J Mol Biol. 2001. PMID: 11292350
-
A credit-card library approach for disrupting protein-protein interactions.Bioorg Med Chem. 2006 Apr 15;14(8):2660-73. doi: 10.1016/j.bmc.2005.11.052. Epub 2005 Dec 27. Bioorg Med Chem. 2006. PMID: 16384710
-
Function of the c-Myc oncogenic transcription factor.Exp Cell Res. 1999 Nov 25;253(1):63-77. doi: 10.1006/excr.1999.4686. Exp Cell Res. 1999. PMID: 10579912 Review.
-
Small-molecule modulators of c-Myc/Max and Max/Max interactions.Curr Top Microbiol Immunol. 2011;348:139-49. doi: 10.1007/82_2010_90. Curr Top Microbiol Immunol. 2011. PMID: 20680803 Review.
Cited by
-
Disruption of Myc-Max heterodimerization with improved cell-penetrating analogs of the small molecule 10074-G5.Oncotarget. 2013 Jun;4(6):936-47. doi: 10.18632/oncotarget.1108. Oncotarget. 2013. PMID: 23801058 Free PMC article.
-
Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase.Proc Natl Acad Sci U S A. 2006 Jan 31;103(5):1289-94. doi: 10.1073/pnas.0510772103. Epub 2006 Jan 23. Proc Natl Acad Sci U S A. 2006. PMID: 16432180 Free PMC article.
-
Protein recognition using synthetic surface-targeted agents.Mol Divers. 2004;8(2):89-100. doi: 10.1023/b:modi.0000025652.55320.16. Mol Divers. 2004. PMID: 15209160 Review.
-
MAX Functions as a Tumor Suppressor and Rewires Metabolism in Small Cell Lung Cancer.Cancer Cell. 2020 Jul 13;38(1):97-114.e7. doi: 10.1016/j.ccell.2020.04.016. Epub 2020 May 28. Cancer Cell. 2020. PMID: 32470392 Free PMC article.
-
Small molecules targeting c-Myc oncogene: promising anti-cancer therapeutics.Int J Biol Sci. 2014 Sep 13;10(10):1084-96. doi: 10.7150/ijbs.10190. eCollection 2014. Int J Biol Sci. 2014. PMID: 25332683 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

