Changes in actin and myosin structural dynamics due to their weak and strong interactions
- PMID: 11892285
- PMCID: PMC10712373
- DOI: 10.1007/978-3-540-46558-4_2
Changes in actin and myosin structural dynamics due to their weak and strong interactions
Abstract
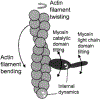
Figure 3 summarizes the effects of actomyosin binding on the internal and global dynamics of either protein, as discussed in this chapter. These effects depend primarily on the strength of the interaction; which in turn depends on the state of the nucleotide at the myosin active site. When either no nucleotide or ADP is bound, the interaction is strong and the effect on each protein is maximal. When the nucleotide is ATP or ADP.Pi, or the equivalent nonhydrolyzable analogs, the interaction is weak and the effect on molecular dynamics of each protein is minimal. The weaker effects in weak-binding states are not simply the reflection of lower occupancy of binding sites--the molecular models in Fig. 3 illustrate the effects of the formation of the ternary complex, after correction for the free actin and myosin in the system. Thus EPR on myosin (Berger and Thomas 1991; Thomas et al. 1995) and pyrene fluorescence studies on actin (Geeves 1991) have shown that the formation of a ternary complex has a negligible effect on the internal dynamics of both [figure: see text] proteins (left side of Fig. 3, white arrows). As shown by both EPR (Baker et al. 1998; Roopnarine et al. 1998) and phosphorescence (Ramachandran and Thomas 1999), both domains of myosin are dynamically disordered in weak-binding states, and this is essentially unaffected by the formation of the ternary complex (left side of Fig. 3, indicated by disordered myosin domains). The only substantial effect of the formation of the weak interaction that has been reported is the EPR-detected (Ostap and Thomas 1991) restriction of the global dynamics of actin upon weak myosin binding (left column of Fig. 3, gray arrow). The effects of strong actomyosin formation are much more dramatic. While substantial rotational dynamics, both internal and global, exist in both myosin and actin in the presence of ADP or the absence of nucleotides, spin label EPR, pyrene fluorescence, and phosphorescence all show dramatic restrictions in these motions upon formation of the strong ternary complex (right column of Fig. 3). One implication of this is that the weak-to-strong transition is accompanied by a disorder-to-order transition in both actin and myosin, and this is itself an excellent candidate for the structural change that produces force (Thomas et al. 1995). Another clear implication is that the crystal structures obtained for isolated myosin and actin are not likely to be reliable representations of structures that exist in ternary complexes of these proteins (Rayment et al. 1993a and 1993b; Dominguez et al. 1998; Houdusse et al. 1999). This is clearly true of the strong-binding states, since the spectroscopic studies indicate consistently that substantial changes occur in both proteins upon strong complex formation. For the weak complexes, the problem is not that complex formation induces large structural changes, but that the structures themselves are dynamically disordered. This is probably why so many different structures have been obtained for myosin S1 with nucleotides bound--each crystal is selecting one of the many different substates represented by the dynamic ensemble. Finally, there is the problem that the structures of actomyosin complexes are probably influenced strongly by their mechanical coupling to muscle protein lattice (Baker at al. 2000). Thus, even if co-crystals of actin and myosin are obtained in the future, an accurate description of the structural changes involved in force generation will require further experiments using site-directed spectroscopic probes of both actin and myosin, in order to detect the structural dynamics of these ternary complexes under physiological conditions.
Figures
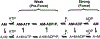
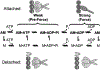
References
-
- Barnett VA, Thomas DD (1987) Resolution of conformational states of spin-labeled myosin during steady-state ATP hydrolysis. Biochemistry 26:314–323 - PubMed
-
- Berger CL, Thomas DD (1991) Rotational dynamics of actin-bound intermediates in the myosin ATPase cycle. Biochemistry 30: 11 036–11 045 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
