Xenopus small heat shock proteins, Hsp30C and Hsp30D, maintain heat- and chemically denatured luciferase in a folding-competent state
- PMID: 11892988
- PMCID: PMC514803
- DOI: 10.1379/1466-1268(2002)007<0006:xshsph>2.0.co;2
Xenopus small heat shock proteins, Hsp30C and Hsp30D, maintain heat- and chemically denatured luciferase in a folding-competent state
Abstract
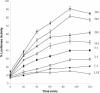
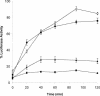
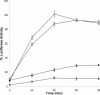
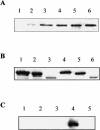
In this study we characterized the chaperone functions of Xenopus recombinant Hsp30C and Hsp30D by using an in vitro rabbit reticulocyte lysate (RRL) refolding assay system as well as a novel in vivo Xenopus oocyte microinjection assay. Whereas heat- or chemically denaturated luciferase (LUC) did not regain significant enzyme activity when added to RRL or microinjected into Xenopus oocytes, compared with native LUC, denaturation of LUC in the presence of Hsp30C resulted in a reactivation of enzyme activity up to 80-100%. Recombinant Hsp30D, which differs from Hsp30C by 19 amino acids, was not as effective as its isoform in preventing LUC aggregation or maintaining it in a folding-competent state. Removal of the first 17 amino acids from the N-terminal region of Hsp30C had little effect on its ability to maintain LUC in a folding-competent state. However, deletion of the last 25 residues from the C-terminal end dramatically reduced Hsp30C chaperone activity. Coimmunoprecipitation and immunoblot analyses revealed that Hsp30C remained associated with heat-denatured LUC during incubation in reticulocyte lysate and that the C-terminal mutant exhibited reduced affinity for unfolded LUC. Finally, we found that Hsc70 present in RRL interacted only with heat-denatured LUC bound to Hsp30C. These findings demonstrate that Xenopus Hsp30 can maintain denatured target protein in a folding-competent state and that the C-terminal end is involved in this function.
Figures






References
-
- Arrigo AP. Small heat shock proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. J Biol Chem. 1998;379:19–26. - PubMed
-
- Arrigo AP, Landry J 1994 Expression and function of the low-molecular weight heat shock proteins. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto RI, Tissieres A, Georgopoulos C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 335–373.
-
- Carver JA, Aquilina JA, Truscott RJW, Ralston GB. Identification by H NMR spectroscopy of flexible C-terminal extensions in bovine lens alpha-crystallin. FEBS Lett. 1992;311:143–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
