NF-kappa B DNA-binding activity in embryos responding to a teratogen, cyclophosphamide
- PMID: 11893254
- PMCID: PMC84630
- DOI: 10.1186/1471-213x-2-2
NF-kappa B DNA-binding activity in embryos responding to a teratogen, cyclophosphamide
Abstract
Background: The Rel/NF-kappaB transcription factors have been shown to regulate apoptosis in different cell types, acting as inducers or blockers in a stimuli- and cell type-dependent fashion. One of the Rel/NF-kappaB subunits, RelA, has been shown to be crucial for normal embryonic development, in which it functions in the embryonic liver as a protector against TNFalpha-induced physiological apoptosis. This study assesses whether NF-kappaB may be involved in the embryo's response to teratogens. Fot this, we evaluated how NF-KappaB DNA binding activity in embryonic organs demonstrating differential sensitivity to a reference teratogen, cyclophosphamide, correlates with dysmorphic events induced by the teratogen at the cellular level (excessive apoptosis) and at the organ level (structural anomalies).
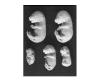
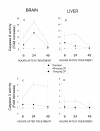
Results: The embryonic brain and liver were used as target organs. We observed that the Cyclophosphamide-induced excessive apoptosis in the brain, followed by the formation of severe craniofacial structural anomalies, was accompanied by suppression of NF-kappaB DNA-binding activity as well as by a significant and lasting increase in the activity of caspases 3 and 8. However, in the liver, in which cyclophosphamide induced transient apoptosis was not followed by dysmorphogenesis, no suppression of NF-kappaB DNA-binding activity was registered and the level of active caspases 3 and 8 was significantly lower than in the brain. It has also been observed that both the brain and liver became much more sensitive to the CP-induced teratogenic insult if the embryos were exposed to a combined treatment with the teratogen and sodium salicylate that suppressed NF-kappaB DNA-binding activity in these organs.
Conclusion: The results of this study demonstrate that suppression of NF-kappaB DNA-binding activity in embryos responding to the teratogenic insult may be associated with their decreased resistance to this insult. They also suggest that teratogens may suppress NF-kappaB DNA-binding activity in the embryonic tissues in an organ type- and dose-dependent fashion.
Figures




References
-
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. - PubMed
-
- Sadler TW, Hunter ES., III . Principles of abnormal development. Past, present and future. In: Kimmel CA, Buelke-Sam J, editor. Developmental Toxicology. New York, Raven Press; 1994. pp. 53–63.
-
- Knudsen TB. Cell death. In: Kavlock RJ, Daston GP, editor. Drug Toxicity in Embryonic Development I. Berlin, Heidelberg, Springer-Verlag; 1997. pp. 211–244.
-
- Green DR. Apoptotic pathways: the roads to ruin. Cell. 1998;94:695–698. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

