Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation
- PMID: 11893586
- PMCID: PMC3918411
- DOI: 10.1152/ajpheart.00645.2001
Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation
Abstract
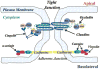
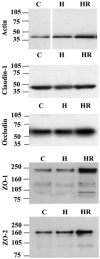
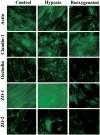
Cerebral microvessel endothelial cells that form the blood-brain barrier (BBB) have tight junctions (TJ) that are critical for maintaining brain homeostasis and low permeability. Both integral (claudin-1 and occludin) and membrane-associated zonula occluden-1 and -2 (ZO-1 and ZO-2) proteins combine to form these TJ complexes that are anchored to the cytoskeletal architecture (actin). Disruptions of the BBB have been attributed to hypoxic conditions that occur with ischemic stroke, pathologies of decreased perfusion, and high-altitude exposure. The effects of hypoxia and posthypoxic reoxygenation in cerebral microvasculature and corresponding cellular mechanisms involved in disrupting the BBB remain unclear. This study examined hypoxia and posthypoxic reoxygenation effects on paracellular permeability and changes in actin and TJ proteins using primary bovine brain microvessel endothelial cells (BBMEC). Hypoxia induced a 2.6-fold increase in [(14)C]sucrose, a marker of paracellular permeability. This effect was significantly reduced (~58%) with posthypoxic reoxygenation. After hypoxia and posthypoxic reoxygenation, actin expression was increased (1.4- and 2.3-fold, respectively). Whereas little change was observed in TJ protein expression immediately after hypoxia, a twofold increase in expression was seen with posthypoxic reoxygenation. Furthermore, immunofluorescence studies showed alterations in occludin, ZO-1, and ZO-2 protein localization during hypoxia and posthypoxic reoxygenation that correlate with the observed changes in BBMEC permeability. The results of this study show hypoxia-induced changes in paracellular permeability may be due to perturbation of TJ complexes and that posthypoxic reoxygenation reverses these effects.
Figures
References
-
- Abbruscato TJ, Davis TP. Combination of hypoxia/aglycemia compromises in vitro blood-brain barrier integrity. J Pharmacol Exp Ther. 1999;289:668–675. - PubMed
-
- Al-Haboubi HA, Ward BJ. Microvascular permeability of the isolated rat heart to various solutes in well-oxygenated and hypoxic conditions. Int J Microcirc Clin Exp. 1996;16:291–301. - PubMed
-
- Archer SL, Freude KA, Shultz PJ. Effect of graded hypoxia on the induction and function of inducible nitric oxide synthase in rat mesangial cells. Circ Res. 1995;77:21–28. - PubMed
-
- Audus KL, Borchardt RT. Bovine brain microvessel endothelial cell monolayers as a model for the blood-brain barrier. Ann NY Acad Sci. 1987;507:9–18. - PubMed
-
- Audus KL, Borchardt RT. Transport of macromolecules across the capillary endothelium. In: Juliano RL, editor. Targeted Drug Delivery. New York: Springer-Verlag; 1991. pp. 43–70.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

