Carcinoembryonic antigen family receptor recognition by gonococcal Opa proteins requires distinct combinations of hypervariable Opa protein domains
- PMID: 11895933
- PMCID: PMC127850
- DOI: 10.1128/IAI.70.4.1715-1723.2002
Carcinoembryonic antigen family receptor recognition by gonococcal Opa proteins requires distinct combinations of hypervariable Opa protein domains
Abstract
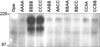
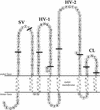
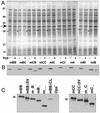
Neisserial Opa proteins function as a family of adhesins that bind heparan sulfate proteoglycan (HSPG) or carcinoembryonic antigen family (CEACAM) receptors on human host cells. In order to define the CEACAM binding domain on Opa proteins, we tested the binding properties of a series of gonococcal (strain MS11) recombinants producing mutant and chimeric Opa proteins with alterations in one or more of the four surface-exposed loops. Mutagenesis demonstrated that the semivariable domain, present in the first loop, was completely dispensable for CEACAM binding. In contrast, the two hypervariable (HV) regions present in the second and third loops were essential for binding; deletion of either domain resulted in loss of receptor recognition. Deletion of the fourth loop resulted in a severe decrease in Opa expression at the cell surface and could therefore not be tested for CEACAM binding. Chimeric Opa variants, containing combinations of HV regions derived from different CEACAM binding Opa proteins, lost most of their receptor binding activity. Some chimeric variants gained HSPG binding activity. Together, our results indicate that full recognition of CEACAM receptors by Opa proteins requires a highly coordinate interplay between both HV regions. Furthermore, shuffling of HV regions may result in novel HSPG receptor binding activity.
Figures


References
-
- Beauchemin, N., P. Draber, G. Dveksler, P. Gold, S. D. Gray-Owen, F. Grunert, S. Hammarstrom, K. V. Holmes, A. Karlsson, M. Kuroki, S. H. Lin, L. Lucka, S. M. Najjar, M. Neumaier, B. Obrink, J. E. Shively, K. M. Skubitz, C. P. Stanners, P. Thomas, J. A. Thompson, M. Virji, S. von Kleist, C. Wagener, S. M. Watt, and W. Zimmermann. 1999. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp. Cell Res. 252:243-249. - PubMed
-
- Belland, R. J., T. Chen, J. Swanson, and S. H. Fischer. 1992. Human neutrophil response to recombinant neisserial Opa proteins. Mol. Microbiol. 6:1729-1737. - PubMed
-
- Berling, B., F. Kolbinger, F. Grunert, J. A. Thompson, F. Brombacher, F. Buchegger, S. von Kleist, and W. Zimmermann. 1990. Cloning of a carcinoembryonic antigen family member expressed in leukocytes of chronic myeloid leukemia patients and bone marrow. Cancer Res. 50:6534-6539. - PubMed
-
- Bhat, K. S., C. P. Gibbs, O. Barrera, S. G. Morrison, F. Jahnig, A. Stern, E. M. Kupsch, T. F. Meyer, and J. Swanson. 1991. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol. Microbiol. 5:1889-1901. - PubMed
-
- Billker, O., A. Popp, S. D. Gray-Owen, and T. F. Meyer. 2000. The structural basis of CEACAM-receptor targeting by neisserial Opa proteins. Trends Microbiol. 8:258-260. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

