Purification and characterization of arginine carboxypeptidase produced by Porphyromonas gingivalis
- PMID: 11895942
- PMCID: PMC127852
- DOI: 10.1128/IAI.70.4.1807-1815.2002
Purification and characterization of arginine carboxypeptidase produced by Porphyromonas gingivalis
Abstract
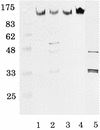
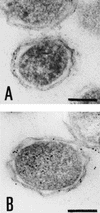
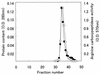
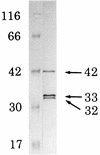
Arginine carboxypeptidase was isolated from the cytoplasm of Porphyromonas gingivalis 381 and purified by DEAE-Sephacel column chromatography, followed by high-performance liquid chromatography on DEAE-5PW and TSK G2000SW(XL). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the purified enzyme revealed the presence of three major bands at 42, 33, and 32 kDa with identical N-terminal sequences. By Western blotting analysis and immunoelectron microscopy, the arginine carboxypeptidase was found to be widely distributed in the cytoplasm and on the surface of the outer membrane. The open reading frame corresponding to the N-terminal amino acids of the arginine carboxypeptidase was detected by a search of the sequence of the P. gingivalis W83 genome. This sequence showed homology with mammalian carboxypeptidases (M, N, and E/H) and included a zinc-binding region signature, suggesting that the enzyme is a member of the zinc carboxypeptidase family. The purified enzyme was inhibited by EGTA, o-phenanthroline, DL-2-mercaptomethyl-3-guanidinoethylthiopropanoic acid, and some metal ions, such as Cu(2+), Zn(2+), and Cd(2+). On the other hand, Co(2+) activated the enzyme. The enzyme released arginine and/or lysine from biologically active peptides containing these amino acids at the C terminus but did not cleave substrates when proline was present at the penultimate position. These results indicate that the arginine carboxypeptidase produced by P. gingivalis is an exo type of metallocarboxypeptidase. This enzyme may function to release arginine in collaboration with an arginine aminopeptidase, e.g., Arg-gingipain, to obtain specific amino acids from host tissues during the growth of P. gingivalis.
Figures

Similar articles
-
CPG70 is a novel basic metallocarboxypeptidase with C-terminal polycystic kidney disease domains from Porphyromonas gingivalis.J Biol Chem. 2002 Jun 28;277(26):23433-40. doi: 10.1074/jbc.M200811200. Epub 2002 Apr 25. J Biol Chem. 2002. PMID: 11976326
-
Purification of a human urinary carboxypeptidase (kininase) distinct from carboxypeptidases A, B, or N.Anal Biochem. 1984 Aug 1;140(2):520-31. doi: 10.1016/0003-2697(84)90203-3. Anal Biochem. 1984. PMID: 6486437
-
Biochemical characterization of the arginine-specific proteases of Porphyromonas gingivalis W50 suggests a common precursor.Biochem J. 1997 May 1;323 ( Pt 3)(Pt 3):701-9. doi: 10.1042/bj3230701. Biochem J. 1997. PMID: 9169603 Free PMC article.
-
The multiple forms of trypsin-like activity present in various strains of Porphyromonas gingivalis are due to the presence of either Arg-gingipain or Lys-gingipain.Infect Immun. 1995 Apr;63(4):1176-82. doi: 10.1128/iai.63.4.1176-1182.1995. Infect Immun. 1995. PMID: 7890369 Free PMC article.
-
Isolation and characterization of a basic carboxypeptidase from human seminal plasma.Arch Biochem Biophys. 1988 Dec;267(2):660-7. doi: 10.1016/0003-9861(88)90074-4. Arch Biochem Biophys. 1988. PMID: 3214176
Cited by
-
Arginine deiminase inhibits Porphyromonas gingivalis surface attachment.Microbiology (Reading). 2013 Feb;159(Pt 2):275-285. doi: 10.1099/mic.0.062695-0. Epub 2012 Dec 14. Microbiology (Reading). 2013. PMID: 23242802 Free PMC article.
-
Porphyromonas gingivalis: keeping the pathos out of the biont.J Oral Microbiol. 2013;5. doi: 10.3402/jom.v5i0.19804. Epub 2013 Apr 3. J Oral Microbiol. 2013. PMID: 23565326 Free PMC article.
-
Role of gingipains in growth of Porphyromonas gingivalis in the presence of human serum albumin.Infect Immun. 2001 Aug;69(8):5166-72. doi: 10.1128/IAI.69.8.5166-5172.2001. Infect Immun. 2001. PMID: 11447200 Free PMC article.
-
Human and monkey lenses cultured with calcium ionophore form alphaB-crystallin lacking the C-terminal lysine, a prominent feature of some human cataracts.Invest Ophthalmol Vis Sci. 2009 Dec;50(12):5828-36. doi: 10.1167/iovs.09-4015. Epub 2009 Jul 15. Invest Ophthalmol Vis Sci. 2009. PMID: 19608539 Free PMC article.
-
Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: implications for autoimmunity in rheumatoid arthritis.Arthritis Rheum. 2010 Sep;62(9):2662-72. doi: 10.1002/art.27552. Arthritis Rheum. 2010. PMID: 20506214 Free PMC article.
References
-
- Banbila, A., P. Mak, M. Bugno, J. Silberring, A. Dubin, D. Nelson, J. Travis, and J. Potempa. 1999. Prolyl tripeptidyl peptidase from Porphyromonas gingivalis. J. Biol. Chem. 274:9246-9252. - PubMed
-
- Barrett, A. J., N. D. Rawling, and J. F. Woessner (ed.). 1998. Handbook of proteolytic enzymes, p. 1318-1351. Academic Press, London, England.
-
- Birkedal, H., R. E. Taylar, J. J. Zambor, P. K. Barwa, and M. E. Neiders. 1988. Characterization of collagenolytic activity from strains of Bacteroides gingivalis. J. Periodontal Res. 23:258-264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

