Entamoeba histolytica-induced dephosphorylation in host cells
- PMID: 11895943
- PMCID: PMC127861
- DOI: 10.1128/IAI.70.4.1816-1823.2002
Entamoeba histolytica-induced dephosphorylation in host cells
Abstract
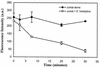
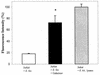
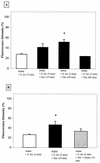
Activation of host cell protein tyrosine phosphatases (PTPases) and protein dephosphorylation is an important mechanism used by various microorganisms to deactivate or kill host defense cells. To determine whether protein tyrosine dephosphorylation played a role in signaling pathways affecting Entamoeba histolytica-mediated host cell killing, we investigated the involvement of PTPases during the attachment of E. histolytica to target cells. We observed a rapid decrease in cellular protein tyrosine levels in Jurkat cells, as measured with an antiphosphotyrosine monoclonal antibody, following adherence to E. histolytica. Ameba-induced protein dephosphorylation was contact dependent and required intact parasite, since blocking amebic adherence with galactose inhibited tyrosine dephosphorylation and amebic lysates had no effect on phosphotyrosine levels. Moreover, disruption of amebic adherence with galactose promoted recovery of phosphorylation in Jurkat cells, indicating that dephosphorylation precedes target cell death. The evidence suggests that ameba-induced dephosphorylation is mediated by host cell phosphatases. Prior treatment of Jurkat cells with phenylarsine oxide, a PTPase inhibitor, inhibited ameba-induced dephosphorylation. We also found proteolytic cleavage of the PTPase 1B (PTP1B) in Jurkat cells after contact with amebae. The calcium-dependent protease calpain is responsible for PTP1B cleavage and enzymatic activation. Pretreatment of Jurkat cells with calpeptin, a calpain inhibitor, blocked PTP1B cleavage and inhibited ameba-induced dephosphorylation. In addition, inhibition of Jurkat cell PTPases with phenylarsine oxide blocked Jurkat cell apoptosis induced by E. histolytica. These results suggest that E. histolytica-mediated host cell death occurs by a mechanism that involves PTPase activation.
Figures






Similar articles
-
Calpain-dependent cleavage of SHP-1 and SHP-2 is involved in the dephosphorylation of Jurkat T cells induced by Entamoeba histolytica.Parasite Immunol. 2010 Mar;32(3):176-83. doi: 10.1111/j.1365-3024.2009.01175.x. Parasite Immunol. 2010. PMID: 20398180
-
Calpain-dependent calpastatin cleavage regulates caspase-3 activation during apoptosis of Jurkat T cells induced by Entamoeba histolytica.Int J Parasitol. 2007 Sep;37(11):1209-19. doi: 10.1016/j.ijpara.2007.03.011. Epub 2007 Apr 6. Int J Parasitol. 2007. PMID: 17498717
-
Apoptotic killing and phagocytosis of host cells by the parasite Entamoeba histolytica.Infect Immun. 2003 Feb;71(2):964-72. doi: 10.1128/IAI.71.2.964-972.2003. Infect Immun. 2003. PMID: 12540579 Free PMC article.
-
Microbes and microbial toxins: paradigms for microbial-mucosal interactions. VI. Entamoeba histolytica: parasite-host interactions.Am J Physiol Gastrointest Liver Physiol. 2001 Jun;280(6):G1049-54. doi: 10.1152/ajpgi.2001.280.6.G1049. Am J Physiol Gastrointest Liver Physiol. 2001. PMID: 11352795 Review.
-
Entamoeba histolytica activates host cell caspases during contact-dependent cell killing.Curr Top Microbiol Immunol. 2005;289:175-84. doi: 10.1007/3-540-27320-4_8. Curr Top Microbiol Immunol. 2005. PMID: 15791956 Review.
Cited by
-
Entamoeba histolytica induces cell death of HT29 colonic epithelial cells via NOX1-derived ROS.Korean J Parasitol. 2013 Feb;51(1):61-8. doi: 10.3347/kjp.2013.51.1.61. Epub 2013 Feb 18. Korean J Parasitol. 2013. PMID: 23467460 Free PMC article.
-
Chew on this: amoebic trogocytosis and host cell killing by Entamoeba histolytica.Trends Parasitol. 2015 Sep;31(9):442-52. doi: 10.1016/j.pt.2015.05.003. Epub 2015 Jun 9. Trends Parasitol. 2015. PMID: 26070402 Free PMC article. Review.
-
A Sequential Model of Host Cell Killing and Phagocytosis by Entamoeba histolytica.J Parasitol Res. 2011;2011:926706. doi: 10.1155/2011/926706. Epub 2011 Jan 20. J Parasitol Res. 2011. PMID: 21331284 Free PMC article.
-
Signaling Role of NADPH Oxidases in ROS-Dependent Host Cell Death Induced by Pathogenic Entamoeba histolytica.Korean J Parasitol. 2022 Jun;60(3):155-161. doi: 10.3347/kjp.2022.60.3.155. Epub 2022 Jun 30. Korean J Parasitol. 2022. PMID: 35772733 Free PMC article. Review.
-
Tissue destruction and invasion by Entamoeba histolytica.Trends Parasitol. 2011 Jun;27(6):254-63. doi: 10.1016/j.pt.2011.02.006. Epub 2011 Mar 26. Trends Parasitol. 2011. PMID: 21440507 Free PMC article. Review.
References
-
- Ariyoshi, H., A. Oda, and E. W. Salzman. 1995. Participation of calpain in protein-tyrosine phosphorylation and dephosphorylation in human blood platelets. Arterioscler. Thromb. Vasc. Biol. 15:511-514. - PubMed
-
- Blanchette, J., N. Racette, R. Faure, K. A. Siminovitch, and M. Olivier. 1999. Leishmania-induced increases in activation of macrophage SHP-1 tyrosine phosphatase are associated with impaired IFN-γ-triggered JAK2 activation. Eur. J. Immunol. 29:3737-3744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

