Inactivation of the Moraxella catarrhalis superoxide dismutase SodA induces constitutive expression of iron-repressible outer membrane proteins
- PMID: 11895952
- PMCID: PMC127887
- DOI: 10.1128/IAI.70.4.1889-1895.2002
Inactivation of the Moraxella catarrhalis superoxide dismutase SodA induces constitutive expression of iron-repressible outer membrane proteins
Abstract
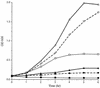
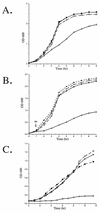
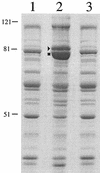
Many pathogens produce one or more superoxide dismutases (SODs), enzymes involved in the detoxification of endogenous and exogenous reactive oxygen species that are encountered during the infection process. One detectable cytoplasmic SOD was identified in the human mucosal pathogen Moraxella catarrhalis, and the gene responsible for the SOD activity, sodA, was isolated from a recent pediatric clinical isolate (strain 7169). Sequence analysis of the cloned M. catarrhalis 7169 DNA fragment revealed an open reading frame of 618 bp encoding a polypeptide of 205 amino acids with 48 to 67% identity to known bacterial manganese-cofactored SODs. An isogenic M. catarrhalis sodA mutant was constructed in strain 7169 by allelic exchange. In contrast to the wild-type 7169, the 7169::sodK20 mutant was severely attenuated for aerobic growth, even in rich medium containing supplemental amino acids, and exhibited extreme sensitivity to the redox-active agent methyl viologen. The ability of recombinant SodA to rescue the aerobic growth defects of E. coli QC774, a sodA sodB-deficient mutant, demonstrated the functional expression of SOD activity by cloned M. catarrhalis sodA. Indirect SOD detection assays were used to visualize both native and recombinant SodA activity in bacterial lysates. This study demonstrates that M. catarrhalis SodA plays a critical role in the detoxification of endogenous, metabolically produced oxygen radicals. In addition, the outer membrane protein (OMP) profile of 7169::sodK20 was consistent with iron starvation in spite of growth under iron-replete conditions. This novel observation indicates that M. catarrhalis strains lacking SodA constitutively express immunogenic OMPs previously described as iron repressible, and this potentially attenuated mutant strain may be an attractive vaccine candidate.
Figures



References
-
- Battistoni, A., F. Pacello, S. Folcarelli, M. Ajello, G. Donnarumma, R. Greco, M. G. Ammendolia, D. Touati, G. Rotilio, and P. Valenti. 2000. Increased expression of periplasmic Cu,Zn superoxide dismutase enhances survival of Escherichia coli invasive strains within nonphagocytic cells. Infect. Immun. 68:30-37. - PMC - PubMed
-
- Beauchamp, C., and I. Fridovich. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44:276-287. - PubMed
-
- Beck, B. L., L. B. Tabatabai, and J. E. Mayfield. 1990. A protein isolated from Brucella abortus is a Cu-Zn superoxide dismutase. Biochemistry 29:372-376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

