Role of various enterotoxins in Aeromonas hydrophila-induced gastroenteritis: generation of enterotoxin gene-deficient mutants and evaluation of their enterotoxic activity
- PMID: 11895956
- PMCID: PMC127858
- DOI: 10.1128/IAI.70.4.1924-1935.2002
Role of various enterotoxins in Aeromonas hydrophila-induced gastroenteritis: generation of enterotoxin gene-deficient mutants and evaluation of their enterotoxic activity
Abstract
Three enterotoxins from the Aeromonas hydrophila diarrheal isolate SSU have been molecularly characterized in our laboratory. One of these enterotoxins is cytotoxic in nature, whereas the other two are cytotonic enterotoxins, one of them heat labile and the other heat stable. Earlier, by developing an isogenic mutant, we demonstrated the role of a cytotoxic enterotoxin in causing systemic infection in mice. In the present study, we evaluated the role of these three enterotoxins in evoking diarrhea in a murine model by developing various combinations of enterotoxin gene-deficient mutants by marker-exchange mutagenesis. A total of six isogenic mutants were prepared in a cytotoxic enterotoxin gene (act)-positive or -negative background strain of A. hydrophila. We developed two single knockouts with truncation in either the heat-labile (alt) or the heat-stable (ast) cytotonic enterotoxin gene; three double knockouts with truncations of genes encoding (i) alt and ast, (ii) act and alt, and (iii) act and ast genes; and a triple-knockout mutant with truncation in all three genes, act, alt, and ast. The identity of these isogenic mutants developed by double-crossover homologous recombination was confirmed by Southern blot analysis. Northern and Western blot analyses revealed that the expression of different enterotoxin genes in the mutants was correspondingly abrogated. We tested the biological activity of these mutants in a diet-restricted and antibiotic-treated mouse model with a ligated ileal loop assay. Our data indicated that all of these mutants had significantly reduced capacity to evoke fluid secretion compared to that of wild-type A. hydrophila; the triple-knockout mutant failed to induce any detectable level of fluid secretion. The biological activity of selected A. hydrophila mutants was restored after complementation. Taken together, we have established a role for three enterotoxins in A. hydrophila-induced gastroenteritis in a mouse model with the greatest contribution from the cytotoxic enterotoxin Act, followed by the Alt and Ast cytotonic enterotoxins.
Figures



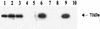
References
-
- Alavandi, S. V., M. S. Subashini, and S. Ananthan. 1999. Occurrence of haemolytic and cytotoxic Aeromonas species in domestic water supplies in Chennai. Indian J. Med. Res. 110:50-55. - PubMed
-
- Albert, M. J., M. Ansaruzzaman, K. Talukdar, A. K. Chopra, I. Kuhn, M. Rahman, A. S. G. Faruque, M. S. Islam, R. B. Sack, and R. Mollby. 2000. Prevalence of enterotoxin genes in Aeromonas spp. isolated from children with diarrhea, healthy controls, and the environment. J. Clin. Microbiol. 38:3785-3790. - PMC - PubMed
-
- Altwegg, M., L. G. Martinetti, J. Luthy-Hottenstein, and M. Rohrbach. 1991. Aeromonas-associated gastroenteritis after consumption of contaminated shrimp. Eur. J. Clin. Microbiol. Infect. Dis. 10:44-45. - PubMed
-
- Annapurna, E., and S. C. Sanyal. 1977. Enterotoxicity of Aeromonas hydrophila. J. Med. Microbiol. 10:317-323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

