Preexisting inflammation due to Mycobacterium bovis BCG infection differentially modulates T-cell priming against a replicating or nonreplicating immunogen
- PMID: 11895959
- PMCID: PMC127859
- DOI: 10.1128/IAI.70.4.1957-1964.2002
Preexisting inflammation due to Mycobacterium bovis BCG infection differentially modulates T-cell priming against a replicating or nonreplicating immunogen
Abstract
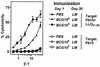
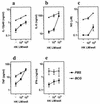
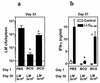
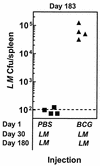
Induction of T-cell memory by vaccination ensures long-term protection against pathogens. We determined whether on-going inflammatory responses during vaccination influenced T-cell priming. A preexposure of mice to Mycobacterium bovis BCG impaired their subsequent ability to prime T cells against Listeria monocytogenes. This was characterized by a decrease in L. monocytogenes-specific gamma interferon (IFN-gamma)-secreting CD4(+) and CD8(+) T cells. The intensity of T-cell priming towards L. monocytogenes depended on the extent of L. monocytogenes expansion, and a cessation of this expansion caused by M. bovis BCG-induced inflammation resulted in impairment in T-cell priming. A challenge of M. bovis BCG-infected mice with a higher L. monocytogenes dose increased L. monocytogenes survival and restored T-cell priming towards L. monocytogenes. Impairment in T-cell priming towards L. monocytogenes due to M. bovis BCG-induced inflammation resulted in a compromised protective efficacy in the long term after mice were rechallenged with L. monocytogenes. Preexisting inflammation selectively impaired T-cell priming for replicating immunogens as CD8(+) T-cell response to ovalbumin administered as an inert antigen (ovalbumin-archaeosomes) was enhanced by M. bovis BCG preimmunization, whereas priming towards ovalbumin administered as a live immunogen (L. monocytogenes-ovalbumin) was impaired. Thus, depending on the nature of the immunogen, the presence of prior inflammatory responses may either impede or boost vaccine efficacy.
Figures



Similar articles
-
A reduced antigen load in vivo, rather than weak inflammation, causes a substantial delay in CD8+ T cell priming against Mycobacterium bovis (bacillus Calmette-Guérin).J Immunol. 2007 Jul 1;179(1):211-20. doi: 10.4049/jimmunol.179.1.211. J Immunol. 2007. PMID: 17579040 Free PMC article.
-
Multiple mechanisms compensate to enhance tumor-protective CD8(+) T cell response in the long-term despite poor CD8(+) T cell priming initially: comparison between an acute versus a chronic intracellular bacterium expressing a model antigen.J Immunol. 2002 Jun 1;168(11):5737-45. doi: 10.4049/jimmunol.168.11.5737. J Immunol. 2002. PMID: 12023374
-
Photochemically-Mediated Inflammation and Cross-Presentation of Mycobacterium bovis BCG Proteins Stimulates Strong CD4 and CD8 T-Cell Responses in Mice.Front Immunol. 2022 Jan 31;13:815609. doi: 10.3389/fimmu.2022.815609. eCollection 2022. Front Immunol. 2022. PMID: 35173729 Free PMC article.
-
T cells and cytokines in intracellular bacterial infections: experiences with Mycobacterium bovis BCG.Ciba Found Symp. 1995;195:123-32; discussion 132-6. doi: 10.1002/9780470514849.ch9. Ciba Found Symp. 1995. PMID: 8724834 Review.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
Cited by
-
Caspase-3 is transiently activated without cell death during early antigen driven expansion of CD8(+) T cells in vivo.PLoS One. 2010 Dec 22;5(12):e15328. doi: 10.1371/journal.pone.0015328. PLoS One. 2010. PMID: 21203525 Free PMC article.
-
Homologous Prime-Boost Vaccination with OVA Entrapped in Self-Adjuvanting Archaeosomes Induces High Numbers of OVA-Specific CD8⁺ T Cells that Protect Against Subcutaneous B16-OVA Melanoma.Vaccines (Basel). 2016 Nov 17;4(4):44. doi: 10.3390/vaccines4040044. Vaccines (Basel). 2016. PMID: 27869670 Free PMC article.
-
XIAP promotes the expansion and limits the contraction of CD8 T cell response through cell extrinsic and intrinsic mechanisms respectively.PLoS Pathog. 2023 Jun 22;19(6):e1011455. doi: 10.1371/journal.ppat.1011455. eCollection 2023 Jun. PLoS Pathog. 2023. PMID: 37347786 Free PMC article.
-
Exposure to mycobacteria primes the immune system for evolutionarily diverse heat shock proteins.Infect Immun. 2005 Nov;73(11):7687-96. doi: 10.1128/IAI.73.11.7687-7696.2005. Infect Immun. 2005. PMID: 16239573 Free PMC article.
-
Transcription factor Batf3 is important for development of CD8+ T-cell response against a phagosomal bacterium regardless of the location of antigen.Immunol Cell Biol. 2016 Apr;94(4):378-87. doi: 10.1038/icb.2015.98. Epub 2015 Dec 8. Immunol Cell Biol. 2016. PMID: 26567886
References
-
- Badovinac, V. P., A. R. Tvinnereim, and J. T. Harty. 2000. Regulation of antigen-specific CD8+ T-cell homeostasis by perforin and interferon-gamma. Science 290:1354-1358. - PubMed
-
- Brunt, L. M., D. A. Portnoy, and E. R. Unanue. 1990. Presentation of Listeria monocytogenes to CD8+ T cells requires secretion of hemolysin and intracellular bacterial growth. J. Immunol. 145:3540-3546. - PubMed
-
- Elkins, K. L., J. T. Rhinehart, S. Stibitz, J. S. Conover, and D. M. Klinman. 1999. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162:2291-2298. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

