Phase I evaluation of delta virG Shigella sonnei live, attenuated, oral vaccine strain WRSS1 in healthy adults
- PMID: 11895966
- PMCID: PMC127867
- DOI: 10.1128/IAI.70.4.2016-2021.2002
Phase I evaluation of delta virG Shigella sonnei live, attenuated, oral vaccine strain WRSS1 in healthy adults
Abstract
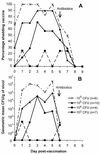
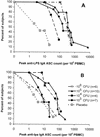
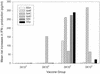
We conducted a phase I trial with healthy adults to evaluate WRSS1, a live, oral Delta virG Shigella sonnei vaccine candidate. In a double-blind, randomized, dose-escalating fashion, inpatient volunteers received a single dose of either placebo (n = 7) or vaccine (n = 27) at 3 x 10(3) CFU (group 1), 3 x 10(4) CFU (group 2), 3 x 10(5) CFU (group 3), or 3 x 10(6) CFU (group 4). The vaccine was generally well tolerated, although a low-grade fever or mild diarrhea occurred in six (22%) of the vaccine recipients. WRSS1 was recovered from the stools of 50 to 100% of the vaccinees in each group. The geometric mean peak anti-lipopolysaccharide responses in groups 1 to 4, respectively, were 99, 39, 278, and 233 for immunoglobulin (IgA) antibody-secreting cell counts; 401, 201, 533, and 284 for serum reciprocal IgG titers; and 25, 3, 489, and 1,092 for fecal IgA reciprocal titers. Postvaccination increases in gamma interferon production in response to Shigella antigens occurred in some volunteers. We conclude that WRSS1 vaccine is remarkably immunogenic in doses ranging from 10(3) to 10(6) CFU but elicits clinical reactions that must be assessed in further volunteer trials.
Figures
References
-
- Black, R. E., M. M. Levine, M. L. Clements, G. Losonsky, D. Herrington, S. Berman, and S. B. Formal. 1987. Prevention of shigellosis by a Salmonella typhi-Shigella sonnei bivalent vaccine. J. Infect. Dis. 155:1260-1265. - PubMed
-
- Cohen, D., S. Ashkenazi, M. S. Green, M. Gdalevich, G. Robin, R. Slepon, M. Yavzori, N. Orr, C. Block, I. Ashkenazi, J. Shemer, D. N. Taylor, T. L. Hale, J. C. Sadoff, D. Pavliakova, R. Schneerson, and J. B. Robbins. 1997. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 349:155-159. - PubMed
-
- Ferreccio, C., V. Prado, A. Ojeda, M. Cayazzo, P. Abrego, L. Guers, and M. M. Levine. 1991. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in a poor periurban setting in Santiago, Chile. Am. J. Epidemiol. 134:614-627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

