Immunodominant epitopes in Babesia bovis rhoptry-associated protein 1 that elicit memory CD4(+)-T-lymphocyte responses in B. bovis-immune individuals are located in the amino-terminal domain
- PMID: 11895969
- PMCID: PMC127881
- DOI: 10.1128/IAI.70.4.2039-2048.2002
Immunodominant epitopes in Babesia bovis rhoptry-associated protein 1 that elicit memory CD4(+)-T-lymphocyte responses in B. bovis-immune individuals are located in the amino-terminal domain
Abstract
Babesia bovis rhoptry-associated protein 1 (RAP-1), which confers partial protection against B. bovis challenge, is recognized by antibodies and T lymphocytes from cattle that have recovered from infection and are immune to subsequent challenge. RAP-1 is a 60-kDa protein with an N-terminal (NT) region that contains four cysteine residues conserved among all Babesia RAP-1 family members and a C-terminal (CT) region that contains multiple, degenerate, tandem 23-amino-acid (aa) repeats. To define the location of CD4(+)-T-cell epitopes for vaccine development using a recombinant protein or minigene construct, a series of truncated recombinant RAP-1 proteins and peptides were tested for stimulation of T-cell lines derived from B. bovis-immune cattle. CD4(+)-T-cell lines from three B. bovis-immune cattle with different DRB3 haplotypes responded to the NT region of RAP-1, whereas T cells from only one animal responded weakly to the CT region. T-cell lines from the three individuals recognized two to six NT-region peptides spanning aa 134 to 316 and representing at least four dominant epitopes. Using RAP-1-specific CD4(+)-T-cell clones, two NT-region epitopes, EYLVNKVLYMATMNYKT (aa 187 to 203) and EAPWYKRWIKKFR (aa 295 to 307), and one CT-region repeat epitope, FREAPQATKHFL, which is present twice at aa positions 391 to 402 and 414 to 425, were identified. Several peptides representing degenerate repeats of the agonist CT-region peptide FREAPQATKHFL neither stimulated responses of T-cell clones specific for this peptide nor inhibited responses to the agonist peptide. Upon stimulation with specific antigen, T-cell clones specific for NT or CT epitopes produced gamma interferon. The presence of T-helper-cell epitopes in the NT domain of RAP-1, which is highly conserved among otherwise antigenically different strains of B. bovis, supports the inclusion of this region in vaccine constructs to be tested in cattle.
Figures

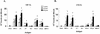


Similar articles
-
Stimulation of T-helper cell gamma interferon and immunoglobulin G responses specific for Babesia bovis rhoptry-associated protein 1 (RAP-1) or a RAP-1 protein lacking the carboxy-terminal repeat region is insufficient to provide protective immunity against virulent B. bovis challenge.Infect Immun. 2003 Sep;71(9):5021-32. doi: 10.1128/IAI.71.9.5021-5032.2003. Infect Immun. 2003. PMID: 12933845 Free PMC article.
-
Babesia bovis rhoptry-associated protein 1 is immunodominant for T helper cells of immune cattle and contains T-cell epitopes conserved among geographically distant B. bovis strains.Infect Immun. 1996 Aug;64(8):3341-50. doi: 10.1128/iai.64.8.3341-3350.1996. Infect Immun. 1996. PMID: 8757873 Free PMC article.
-
Conservation of Babesia bovis small heat shock protein (Hsp20) among strains and definition of T helper cell epitopes recognized by cattle with diverse major histocompatibility complex class II haplotypes.Infect Immun. 2004 Feb;72(2):1096-106. doi: 10.1128/IAI.72.2.1096-1106.2003. Infect Immun. 2004. PMID: 14742557 Free PMC article.
-
Identification of candidate vaccine antigens of bovine hemoparasites Theileria parva and Babesia bovis by use of helper T cell clones.Vet Parasitol. 1995 Mar;57(1-3):189-203. doi: 10.1016/0304-4017(94)03120-l. Vet Parasitol. 1995. PMID: 7597783 Review.
-
Immune control of Babesia bovis infection.Vet Parasitol. 2006 May 31;138(1-2):75-87. doi: 10.1016/j.vetpar.2006.01.041. Epub 2006 Feb 28. Vet Parasitol. 2006. PMID: 16510249 Review.
Cited by
-
In silico and phylogenetic analyses of partial BbRAP-1, BbCP2, BbSBP-4 and BbβTUB gene sequences of Babesia bovis isolates from cattle in South Africa.BMC Vet Res. 2017 Dec 8;13(1):383. doi: 10.1186/s12917-017-1261-7. BMC Vet Res. 2017. PMID: 29216890 Free PMC article.
-
A conserved motif in the immune-subdominant RAP-1 related antigen of Babesia bovis contains a B-cell epitope recognized by antibodies from protected cattle.Front Immunol. 2024 Apr 24;15:1380660. doi: 10.3389/fimmu.2024.1380660. eCollection 2024. Front Immunol. 2024. PMID: 38720894 Free PMC article.
-
Quantitation of Anaplasma marginale major surface protein (MSP)1a and MSP2 epitope-specific CD4+ T lymphocytes using bovine DRB3*1101 and DRB3*1201 tetramers.Immunogenetics. 2006 Sep;58(9):726-39. doi: 10.1007/s00251-006-0140-3. Epub 2006 Aug 19. Immunogenetics. 2006. PMID: 16924490
-
Developing Anti-Babesia bovis Blood Stage Vaccines: A New Perspective Regarding Synthetic Vaccines.Int J Mol Sci. 2023 Mar 9;24(6):5219. doi: 10.3390/ijms24065219. Int J Mol Sci. 2023. PMID: 36982294 Free PMC article. Review.
-
Differentiation and Regulation of Bovine Th2 Cells In Vitro.Cells. 2024 Apr 24;13(9):738. doi: 10.3390/cells13090738. Cells. 2024. PMID: 38727273 Free PMC article.
References
-
- Ababou, A., W. C. Davis, and D. Levy. 1993. The DA6-147 monoclonal antibody raised against the HLA-DR alpha chain identifies a cryptic epitope on the BoLA-DR alpha chain. Vet. Res. 24:402-407. - PubMed
-
- Ababou, A., J. Goyeneche, W. C. Davis, and D. Levy. 1994. Evidence for the expression of three different BoLA-class II molecules on the bovine BL-3 cell line: determination of a non-DR non-DQ gene product. J. Leukoc. Biol. 56:182-186. - PubMed
-
- Beyer, J. C., R. W. Stich, W. C. Brown, and W. P. Cheevers. 1998. Cloning and expression of caprine interferon-gamma. Gene 210:103-108. - PubMed
-
- Bischoff, E., M. Guillotte, O. Mercereau-Puijalon, and S. Bonnefoy. 2000. A member of the Plasmodium falciparum P60 multigene family codes for a nuclear protein expressed by readthrough of an internal stop codon. Mol. Microbiol. 35:1005-1016. - PubMed
-
- Brown, W. C., and D. J. Grab. 1985. Biological and biochemical characterization of bovine interleukin-2. Studies with cloned bovine T cells. J. Immunol. 133:3184-3190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

