Lipoteichoic acids from Lactobacillus johnsonii strain La1 and Lactobacillus acidophilus strain La10 antagonize the responsiveness of human intestinal epithelial HT29 cells to lipopolysaccharide and gram-negative bacteria
- PMID: 11895971
- PMCID: PMC127868
- DOI: 10.1128/IAI.70.4.2057-2064.2002
Lipoteichoic acids from Lactobacillus johnsonii strain La1 and Lactobacillus acidophilus strain La10 antagonize the responsiveness of human intestinal epithelial HT29 cells to lipopolysaccharide and gram-negative bacteria
Abstract
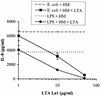
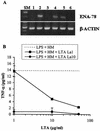
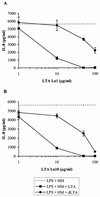
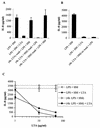
Intestinal epithelial cells (IECs) respond to lipopolysaccharide (LPS) from gram-negative bacteria in the presence of the soluble form of CD14 (sCD14), a major endotoxin receptor. Since sCD14 is also known to interact with gram-positive bacteria and their components, we looked at whether sCD14 could mediate their effects on human IECs. To this end, we examined the production of proinflammatory cytokines following exposure of the IECs to specific gram-positive bacteria or their lipoteichoic acids (LTAs) in the absence and presence of human milk as a source of sCD14. In contrast to LPS from Escherichia coli or Salmonella enteritidis, neither the gram-positive bacteria Lactobacillus johnsonii strain La1 and Lactobacillus acidophilus strain La10 nor their LTAs stimulated IECs, even in the presence of sCD14. However, both LTAs inhibited the sCD14-mediated LPS responsiveness of IECs. We have previously hypothesized that sCD14 in human milk is a means by which the neonate gauges the bacterial load in the intestinal lumen and liberates protective proinflammatory cytokines from IECs. The present observations suggest that gram-positive organisms, via their LTAs, temper this response and prevent an exaggerated inflammatory response.
Figures

Similar articles
-
Cell surface-associated lipoteichoic acid acts as an adhesion factor for attachment of Lactobacillus johnsonii La1 to human enterocyte-like Caco-2 cells.Appl Environ Microbiol. 1999 Mar;65(3):1071-7. doi: 10.1128/AEM.65.3.1071-1077.1999. Appl Environ Microbiol. 1999. PMID: 10049865 Free PMC article.
-
Lipoteichoic acid acts as an antagonist and an agonist of lipopolysaccharide on human gingival fibroblasts and monocytes in a CD14-dependent manner.Infect Immun. 1999 Apr;67(4):1623-32. doi: 10.1128/IAI.67.4.1623-1632.1999. Infect Immun. 1999. PMID: 10084995 Free PMC article.
-
Differential immunostimulatory effects of Gram-positive bacteria due to their lipoteichoic acids.Int Immunopharmacol. 2009 Jan;9(1):127-33. doi: 10.1016/j.intimp.2008.10.014. Epub 2008 Nov 14. Int Immunopharmacol. 2009. PMID: 19013542
-
Binding of bacterial peptidoglycan to CD14.J Biol Chem. 1998 Apr 10;273(15):8680-90. doi: 10.1074/jbc.273.15.8680. J Biol Chem. 1998. PMID: 9535844
-
Targeting aberrant colon cancer-specific DNA methylation with lipoteichoic acid-deficient Lactobacillus acidophilus.Gut Microbes. 2013 Jan-Feb;4(1):84-8. doi: 10.4161/gmic.22822. Epub 2012 Nov 8. Gut Microbes. 2013. PMID: 23137966 Free PMC article. Review.
Cited by
-
Postbiotics: Functional Food Materials and Therapeutic Agents for Cancer, Diabetes, and Inflammatory Diseases.Foods. 2023 Dec 26;13(1):89. doi: 10.3390/foods13010089. Foods. 2023. PMID: 38201117 Free PMC article. Review.
-
Recombinant p40 Protein Promotes Expression of Occludin in HaCaT Keratinocytes: A Brief Communication.Microorganisms. 2023 Dec 3;11(12):2913. doi: 10.3390/microorganisms11122913. Microorganisms. 2023. PMID: 38138057 Free PMC article.
-
Cell surface-associated elongation factor Tu mediates the attachment of Lactobacillus johnsonii NCC533 (La1) to human intestinal cells and mucins.Infect Immun. 2004 Apr;72(4):2160-9. doi: 10.1128/IAI.72.4.2160-2169.2004. Infect Immun. 2004. PMID: 15039339 Free PMC article.
-
Differential inflammatory mediator response in vitro from murine macrophages to lactobacilli and pathogenic intestinal bacteria.Int J Exp Pathol. 2007 Jun;88(3):155-64. doi: 10.1111/j.1365-2613.2007.00530.x. Int J Exp Pathol. 2007. PMID: 17504445 Free PMC article.
-
The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept).Genes Nutr. 2011 Aug;6(3):261-74. doi: 10.1007/s12263-011-0218-x. Epub 2011 Apr 16. Genes Nutr. 2011. PMID: 21499799 Free PMC article.
References
-
- Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. - PubMed
-
- Arakaki, R., S. Sugawara, H. Nakashima, S. Kotani, and H. Takada. 1998. A lipoteichoic acid fraction of Enterococcus hirae activates cultured human monocytic cells via a CD14-independent pathway to promote cytokine production, and the activity is inhibited by serum components. FEMS Immunol. Med. Microbiol. 22:283-291. - PubMed
-
- Bazil, V., V. Horejsi, M. Baudys, H. Kristofova, J. L. Strominger, W. Kostka, and I. Hilgert. 1986. Biochemical characterization of a soluble form of the 53-kDa monocyte surface antigen. Eur. J. Immunol. 16:1583-1589. - PubMed
-
- Beutler, B. 2000. Tlr4: central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 12:20-26. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

